Abstract
Silver and copper nanoparticles (AgNPs and CuNPs) have attracted worldwide attention due to their unique antimicrobial properties. Ethanol and water extracts of hawthorn fruit were chosen to prepare AgNPs and CuNPs. The effects of the pH, contact time, temperature of the solution, type of precursor of metal ions and type of hawthorn extract on the size distribution of particles were studied. The monodispersed and stable AgNPs and CuNPs were prepared with an average size of 60 nm and 200 nm, respectively. Total phenolic content and the anthocyanin content of extracts were determined. The antimicrobial activities of AgNPs and CuNPs were carried out with a series of microbial assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles have unique physical, chemical and biological properties, which makes them applicable in all branches of industry. Especially, due to strong antimicrobial properties, metal nanoparticles have found application in medicine and industrial sectors, where these properties are the most desirable (Mercado et al. 2019; Shaheen et al. 2019). Methods of synthesis of nanomaterials using substances of natural origin have become a popular field of science (Pelle Della and Compagnone 2018). The benefits of such solution are the reduction in use of toxic substances, especially, if organic residues such as pomace and unprocessed leftovers from the food industry are used. The wastes still often contain significant amounts of active substances, and thus they can be reused. The use of natural extracts that simultaneously act as reducer and stabiliser can increase the microbiological activity of nanoproducts, by enhancing the biocidal properties of nanoparticles (Hussain et al. 2016).
In the synthesis of nanoparticles, herbal, fruit, as well as vegetable extracts are widely used (Zain et al. 2014). Silver and copper nanoparticles are broadly used mainly as antibacterial and antifungal agents (Khatoon et al. 2015). Many types of extracts used for synthesis of nanoparticles are known. Ibrahim (2015) synthesised and analysed antimicrobial activity of the silver nanoparticles using banana peel extract. Yasir et al. (2018) used fresh leaves of Syngonium podophyllum for synthesis of silver nanoparticles, whereas Padma et al. (2018) investigated synthesis of copper nanoparticles using extracts from Punica granatum. Depending on the plant’s part, nanomaterials can be characterised by different properties and different biological activity. Even for the same type of plant, nanoparticles with different size and shape can be obtained (Pandurangan et al. 2018). For example, seeds consist of higher concentration of bioactive substances as lignin, saponin and vitamins, whereas fruits can contain higher amounts of flavonoids especially tannins (Kuppusamy et al. 2016). Thakur et al. (2018) investigated effect of root extract and leaf extract of Asparagus adscendens Roxb on synthesis of copper nanoparticles and their antimicrobial activities. Inhibition zone of copper nanoparticles synthesised from root extract was slightly larger compared to inhibition zone of nanocopper from leaf extract.
Plant extracts are associated with the presence of several bioactive compounds as well as carbohydrates, glycosides, alkaloids, polyphenols, terpenoids, ascorbic acid, amino acids, oxalic acid (Della Pelle et al. 2019). Phytochemicals can be responsible for the reduction of metal ions and the stabilisation nanoparticles. Flavonoids are a group of polyphenolic compounds consisting of anthocyanins, isoflavones, flavonols, chalcones, among others. These compounds can stabilise and reduce metal ions to nanoparticles. In addition, the flavonoids contain various functional groups capable of forming nanoparticles (Della Pelle et al. 2018). It has been proposed that tautomeric transformations of flavonoids from the enol form into the keto form can release reactive hydrogen atoms that can reduce metal ions to nanoparticles. However, there is no clear evidence which specific phytochemicals are responsible for the synthesis of nanoparticles (Jeevanandam et al. 2016). The process of obtaining nanoparticles can be divided into three stages: activation phase, growth phase and termination phase (Chokkareddy and Redhi 2018). In the primary stage, biomolecules reduce metal ions and change their monovalent or divalent oxidation states to zero-valent states and nucleation. During the growth of nanoparticles, the presence of plant components affects their shape and final activity. The difference in nanoparticles’ shape and concentration of these energetic biomolecules is thought to be one of the key factors related with the variety of nanoparticle sizes and shapes produced (Mittal et al. 2013). Possible chemical reaction of metal nanoparticles with components of plant extract is presented in Fig. 1.
Hawthorn (Crataegus pontica L.) is considered as one of the oldest traditional medicinal plants due to numerous chemical constituents (Öztürk and Tunçel 2011). The plant is a thorny shrub or small tree with bright green leaves and white flowers. Hawthorn is widely used for preventing and treating cardiovascular diseases. Till now, over 150 active compounds have been isolated from the plant (Kang et al. 2017). Fruits, leaves and flowers contain a number of chemical substances, flavonoids (0.1–2%), oligomeric proanthocyanidins (1–3%), triterpene acids (0.5–1.4%), organic acids (2–6%), among others. Antioxidants present in the plant are responsible for free radical scavenging activity such as epicatechin and chlorogenic acid (Chang et al. 2002).
The aim of the present study is to synthesise silver and copper nanoparticles using hawthorn extracts. The various factors affecting metal ions reduction were investigated. Besides, their antimicrobial activity against microorganisms was investigated.
Materials and methods
Materials
In this study, dried hawthorn berries were purchased at a commercial store. Silver and copper ion solutions were prepared by dissolving AgNO3, Ag(CH3COO), CuSO4 or Cu(CH3COO)2 in deionized water. All reagents used in the study were of analytical grade (Sigma-Aldrich).
Synthesis of AgNPs and CuNPs
In the first part, two types of hawthorn extracts were prepared. For the preparation of ethanol or water hawthorn extracts, dried hawthorn fruits (10 g) were shredded and the berries were extracted in 150 ml of 95% ethanol or in 150 ml of water in a Soxhlet apparatus for 3 h. Each extract was filtered and used in the synthesis of metal nanoparticles.
To obtain silver or copper nanoparticle suspensions, 30 ml of AgNO3, Ag(CH3COO), CuSO4 or Cu(CH3COO)2 solution was mixed with 10 ml of ethanol or water hawthorn extract. Concentration of each metal ions solutions was equal \(133\,{\text{mg}}_{{{\text{Me}}^{n + } }} /l\), so the final concentration of nanoparticles in the suspensions was \(100\,{\text{mg}}_{{{\text{Me}}^{n + } }} /l\). Next, 0.20–0.25 mol/l sodium hydroxide solution was dropped to activate compounds contained in each extract and to establish pH of the solution.
In the study, impacts of type of metal ions precursor, solvent type of extract (water or ethanol hawthorn extract), the pH of the solutions (7–11), the processing time (20–60 min) and the temperature of the process (20–60 °C) were examined on size of the metal nanoparticles. The most favourable values of parameters in reduction processes for each type of metal ions salt and type of extraction were determined using the CCD (central composite design) plans. Summarily, there were 8 plans with 17 experiments. All experimental planning was performed using STATISTICA (version 10.0).
Metal nanoparticles characterization
The solutions of metal nanoparticles with hawthorn extract were characterised using spectroscopic UV–Vis (Rayleigh UV18000 spectrophotometer) and dynamic light scattering (DLS) (Malvern Instruments, ZS-90 and Brookhaven, zetaPALS) methods. Furthermore, size and shape of nanoparticles were examined using scanning electron microscopy technique (SEM) (Vegall-Tescan Company) and scanning transmission electron microscopy (Quanta 3D FEG). The functional groups attached to the nanoparticles surface were detected using an FTIR spectroscope (Nicolet 380), where the spectra were recorded from 4000 to 400 cm−1.
Survival analysis of microorganisms
The prepared sterile growth medium (15 ml) of YPD (yeast extract, casein peptone, sucrose, agar) was added to the Petri dish. Next, 100 μl of the test sample and 25 μl of cell suspension of Aspergillus niger, Escherichia coli or Saccharomyces cerevisiae were added under sterile conditions of the laminar chamber depending on the variant of the experiment. In the next step, the preparation was evenly distributed over the whole surface of the nutrient solution. The samples of cultures prepared in this way were placed in an incubator at 30 °C. After 24 and 48 h, the growth of the microorganisms was evaluated. As reference samples, samples with 100 μl of water or ethanol were used.
Evaluation of the antioxidant content and the antioxidant capacity
In both water and ethanol extracts, the following were determined: total phenolic content (Folin–Ciocalteu method), ferric reducing antioxidant power (FRAP method), total anthocyanin content (Fuleki and Francis method), antioxidant activity due to scavenging free radicals of DPPH and total ascorbic acid content (DCIP method) were determined. All experiments were repeated three times.
Folin–Ciocalteu method—the total phenolic content
To determine the total phenolic content (TPC), the Folin–Ciocalteu method was used. The method is based on the reversible reduction of molybdenum(VI) to molybdenum(V) contained in the Folin–Ciocalteu reagent. The process undergoes in an alkaline environment in the presence of polyphenols (Alkaya et al. 2019). At the beginning, 500 μl of each extract was mixed with 1 ml of Folin–Ciocalteu reagent (previously diluted tenfold by deionised water) and 2 ml of sodium carbonate (75 g/l) solution. The mixture was allowed to stand for 120 min and absorbance was measured at 725 nm. On the basis of the calibration curve showing the dependence of the absorbance of the gallic acid solution on its concentration in the solution, the total phenolic content was determined.
FRAP—ferric reducing antioxidant power
The reducing force was determined by the FRAP method (ferric reducing antioxidant power). In this method, the ability of substance with antioxidant properties to reduce the Fe3+–TPTZ iron complex (ferric-2,4,6-tripyridyl-s-tri-divine complex) to the Fe2+–TPTZ complex was established (Achuba 2018). 10 μl of the extracts was mixed with 2 ml of FRAP reagent. After 30 min, the decrease in the absorbance of the Fe3+–TPTZ complex was measured spectrophotometrically at 593 nm. The antioxidant content in water and ethanol extracts was comparted to activity of the Trolox. A calibration curve was made of the dependence of the absorbance of the Trolox mixture and the FRAP reagent on its concentration \((y = 85.954x - 1.8498,\,R^{2} = 0.9987)\).
The total anthocyanin content—by Fuleki and Francis method
The pH-differential method can be used for the determination of total anthocyanin content (TAC). The method developed by Fuleki and Francis is based on the structural change of the anthocyanin chromophore between pH 1.0 and 4.5 (Fuleki and Francis 1968). Solution of potassium chloride (0.025 M) with chloride acid was used as pH 1.0 buffer and solution of sodium acetate (0.40 M) with chloride acid was used as pH 4.5 buffer. 1 ml of each extract was mixed with 4.5 ml of pH 1.0 buffer and pH 4.5 buffer. The absorbance of prepared mixtures at 520 nm and 700 nm was measured (Kocira et al. 2018). The total anthocyanin content is directly proportional to the difference in absorbance of solutions diluted by buffers:
DPPH—antioxidant activity due to scavenging free radicals
Determination of the free-radical-scavenging assay was carried out using DPPH reagent (1,1-diphenyl-2-picrylhydrazyl). Reduction of stable free radical purple coloured DPPH to the yellow diphenylpicryl hydrazine allows to calculate the inhibition percentage. The higher is the antioxidant activity of the sample, the higher is the reduction of the DPPH radical (Kumar et al. 2018). Absorbance of DPPH solution at 517 nm was measured after 30 min. The antioxidant activity of the extracts was expressed as a percentage reduction of the DPPH radical with the extract relative to the sample without antioxidant active substance:
where I is the DPPH inhibition percentage, Ac the absorbance of the negative control (containing 100 μl of MeOH instead of the samples), As is the absorbance of the samples.
Method DCIP—the total ascorbic acid content
Determination of ascorbic acid content was determined by the oximetry method, which is based on the redox reaction with 2,6-dichlorophenolindophenol. The burette was filled with 0.1% dichlorophenolindophenol. 5 ml of the extract was mixed with 45 ml 2% oxalic acid and 10 ml of mixture was added into the respective beaker for each sample. The dichlorophenolindophenol was then added dropwise, until the blue colour did not disappear and volume used was noted (Anebi et al. 2016). The concentration of DCIP was determined by titration with a solution of sodium thiosulphate. The ascorbic acid content was calculated in mg/g sample (Kumar 2017).
Results
Evaluation of the antioxidant content and the antioxidant capacity
Quantitative analysis of compounds in hawthorn extracts is presented in Table 1. Water extract of hawthorn berries compared to ethanol extract pointed out higher total phenolic content and anthocyanins content. The contents of polyphenols and anthocyanins are directly related to the antioxidant properties. Polyphenols, ascorbic acid and anthocyanins are responsible for the reduction and stabilisation of metal ions in the process of obtaining metal nanoparticles (Della Pelle et al. 2015). Alirezalu et al. (2018) investigated antioxidant activity in flowers and leaves of hawthorn species. The antioxidant activity widely varied in species and in different organs of each individual plant, ranging from 0.9 to 4.65 mmol Fe3+/g in dry weight of the plant, calculated through the FRAP method. The total amount of phenolics ranges from 7.21 to 87.73 mg GAE/g in dry weight of the plant, which is consistent with the obtained results. Concentration of flavonoids in water extraction is about 20% higher compared to ethanol extract. Similarly, ascorbic acid concentration is 1.5 times higher in water extract than in alcohol extract, which can be associated with a lower solubility of ascorbic acid in ethanol solution. Tadić et al. (2008) using HPLC analysis determined free radical scavenging and antimicrobial activities of hawthorn berries ethanol extract. Their analysis showed that the major flavanol components in the extract were hyperoside, vitexin, rutin, isoquercitrin and quercetin. Total flavonoids content was equal to 35.4 ± 2.48. Depending on the extract concentration, its DPPH free radical scavenging activity varied approximately from 90 to 40%. Salmanian et al. (2014) investigated phenolic content and antioxidant properties of hawthorn seed and pulp extracts. Results showed strong antioxidant properties both in pulp and seed extracts. High antioxidant properties were correlated with high level of polyphenols. Using HPLC method, they established content of gallic acid, chlorogenic acid and caffeic acid. In pulp extraction, the concentration of the compounds was equal: 0.022, 0.509 and 0.012 mg/g dried plant. Urbinaviciŭte et al. (2006) determined the content of total flavonoids in hawthorn ethanolic extracts. Extract with different concentration of aqueous ethanol (40–96%v/v) was compared. Results of concentration of three flavonoids: rutin, vitexin-2″-O-rhamnoside, hyperoside and chlorogenic acid in the hawthorn extracts confirmed significant impact of their contents in ethanol solutions Miao et al. (2016) investigated chemical constituents of 80% ethanol, 80% methanol, 80% acetone and pure deionised water extracts of hawthorn fruits. The highest antioxidant activities (established by the DPPH scavenge capacity and ferric reducing power) was obtained in water extract. The researchers confirmed that, flavonoids, polyphenols, vanillic acid, gallic acid, catechin and chlorogenic acid, all contributed to the antioxidant activity.
Impacts of parameters on synthesis of metal nanoparticles
Analysis of the statistical significance of the parameters showed a meaningful impact of the type of extract, temperature and pH of the solution. In the case of process time, the parameter turned out to be important only when creating copper nanoparticles. Table 2 shows the input factors taken into account and the extent of their variation. The mean size of nanoparticles was assumed as the output factor.
Effect of type of extract and type of metal ions precursor
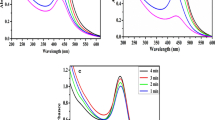
In Fig. 2, UV–Vis absorption spectra of synthesised silver and copper nanoparticles using water and ethanol extracts of hawthorn are presented. The pH value of the solutions affect the localised surface plasmon resonance (LSPR) peak of silver nanoparticles achieved at 390–423 nm (Shrivas et al. 2016). Absorption spectra of AgNPs obtained by alcohol extract were characterised by maximum peak shift towards the longer wavelength as well as higher absorbance compared to AgNPs based on water extract. Similar dependences were observed from the DLS analysis. Statistical analysis showed a significant effect of the silver nanoparticle precursor on the size of the obtained nanoparticles. In the formation of copper nanoparticles, the water-based extract proved better, while for the formation of silver nanoparticles the ethanol extract exhibited better properties. Using the given substances, nanoparticles of a smaller diameter and better homogeneity were obtained. Copper nanoparticles, in a small extent, showed the localised surface plasmon resonance. Despite the preparation of stable nanoparticles, a maximum peak of CuNPs can be detected only in appropriate conditions. The maximum absorbance for CuNPs, depending on the particle properties, is around 600 nm of wavelength (Young-Tae et al. 2019). Figure 2b shows the obtained UV–Vis spectra for CuNPs. The presence of polyphenol compounds affects the colour of the solution. Maximum absorbance at 300 nm can cover LSPR formed from CuNPs. Dang et al. (2011) investigated the formation of copper nanoparticles using various reducing agents (ascorbic acid solution and NaBH4 solution). Only using strong reducing agent when the solution was red, the plasmon absorbance (562 nm) appeared. In the case of obtaining solutions in a different colour (yellow, brown, green), LSPR does not occur.
The high impact of the solvent on the size of the particles being formed was confirmed. The characteristics of both extracts showed a higher concentration of flavonoids for aqueous solutions. CuNPs require strongly stabilising compounds; hence, smaller CuNPs particles were obtained. Silver nanoparticles do not require compounds with strong reducing properties. The high concentration of polyphenols and ascorbic acid can block the formation of large amounts of AgNPs nucleons, hence a significant proportion of larger particles. Fernández-Agulló et al. (2013) analysed the effect of the solvent (water, methanol, ethanol and mixtures of water and alcohol) on the antioxidant and antimicrobial properties of walnut green husk extracts. The highest extraction yield was achieved with water. The optimization of reducing agent’s concentration has also been reported in the literature. The reducing agent affects the size of copper nanoparticle as high concentrations may decrease the size while maintaining the concentration of the precursor. The reducing agent should be at least five times more concentrated compared to the precursor. The nucleation rate is also related to the concentration of the reducing agent. The stabilising agent or the surfactant also affects the size of nanoparticles (Din and Rehan 2017). An important compound found in plants, and, thus, in extracts is carbohydrates. Glucose and others greatly contribute to the reduction of metal ions forming nanoparticles. It has been confirmed that carbohydrates dissolve in water well; however, they have limited solubility in alcohols, including ethanol (Alves et al. 2007). The content of sugars can significantly affect the formation of nanoparticles, including their stability and size (Shakeel et al. 2016). Hence, the smaller size of copper nanoparticles using aqueous extract can be obtained.
Effect of pH
The effect of the pH of the solution was investigated in the range of 7–11. Figure 2 shows the UV–Vis spectra at different pH of silver nanoparticles for water and ethanol extracts. The colour of reaction mixture was pH dependent. Specially, influence of the solution pH was best in the case of silver nanoparticles synthesis from ethanol extract. At neutral condition, AgNPs had the smallest size and were characterised by the highest absorbance on the UV–Vis spectrum. At pH 9 and 11, the particles were characterised by dark brown colour with lower intensity and wider size distribution (Table 2). Ibrahim (2015) obtained the highest absorbance intensity at pH 4.5 for silver nanoparticles synthesised using banana peel extract. On the other hand, the DLS analysis revealed a significant effect of the alkaline environment on the reduction of particle size, regardless of the type of extract and the type of nanoparticles to be formed. In Fig. 3, impact of the pH of solutions on diameter of metal nanoparticles is presented.
Effect of temperature
The temperature also affected the process of reduction, both for silver and copper ions. Reaction mixtures prepared at 20 °C showed light brown colour. At higher reaction temperature, dark reddish-brown colour and more intense SPR peaks were revealed. Size distribution analysis (Fig. 4) presented that the size of metal nanoparticles decreased with the temperature increase, but only when using water extract. At 60 °C, all systems of metal nanoparticles, regardless of the type of salt used, revealed the smallest size. Similarly, results were achieved by Baghizadeh et al. (2015), who synthesised silver nanoparticles using seed extract of Calendula. They confirmed also that the rate of silver nanoparticles formation increased with increasing temperature. Both for silver and copper suspensions, ethanol extract in the lower temperature allowed to achieve smaller particles. Higher temperature could deactivate alcohol extract and decrease stabilising properties of the reagent.
Effect of reduction time
The maximum reduction of metal ions was obtained after 60 min. The rapid generation of nanoparticles resulted from the excellent reducing potential of the active components of hawthorn extracts and their stabilising properties (Fig. 5).
Table 3 presents systems of silver and copper nanoparticles with the smallest particle size distribution and parameters of the processes. From the analysis of zeta potential, solutions with high stability were as follows: Cu(CH3COO)2—water extract, CuSO4—water extract as well as Ag(CH3COO)—ethanol extract and AgNO3—ethanol extract. Presented mixtures were also characterised by the highest homogeneity (Fig. 6).
SEM and STEM analysis of silver and copper nanoparticles
The surface morphology of prepared silver and copper nanoparticles were analysed using SEM. Figure 7 presents silver nanoparticles and copper nanoparticles obtained with the use of water and ethanol extracts of hawthorn. A greater degree of agglomeration was observed in the suspensions of nanoparticles derived from acetate salts, which indicates lower stability of the system. In the case of nanoparticles derived from AgNO3 and CuSO4 salts, smaller particles were obtained. However, particles with a higher dispersion and more regular shape were obtained for the nanoparticles synthesised in the alcohol extract, which may indicate that ethanol improves the system stability and creates symmetrical nanoparticles. STEM micrographs confirmed agglomeration of AgNPs and CuNPs, which may also affect their antimicrobial properties (Fig. 8). In both the cases, nanoparticles with high homogeneity were obtained. Cui et al. (2018) applied hawthorn fruit extract to green synthesis of selenium nanoparticles. They obtained monodispersed and stable SeNPs with an average size of 113 nm.
FTIR analysis of silver and copper nanoparticles
Fourier transform infrared spectroscopy (FTIR) was used to identify the possible biomolecules which are responsible for the reduction and stabilisation of AgNPs and CuNPs. Figure 9 presents the FTIR spectra of AgNPs and CuNPs nanoparticles synthesised using water and ethanol extracts of hawthorn. Metal nanoparticles were synthesised using AgNO3 and CuSO4. The major spectra show bands at 3300, 2914, 2850, 1740, 1600, 1435, 1400, 1295, 1055, 1030 and 875 cm−1 (Fig. 9). The strong infrared band near 3300 cm−1 was observed for the O–H stretching vibration of hydroxyl group. The absorption peak at 2914 cm−1 might be induced by C–H stretching vibration of the CH2 and CH3 groups (Pasandide et al. 2017). The absorption at 1740 cm−1 was caused by C=O stretching vibration, while the absorption at about 1625–1600 cm−1 was due to the C=O asymmetrical stretching vibration of free carboxyl groups typical for the structure of flavonoids. The group of peaks in the range of 1600–1400 cm−1 corresponds to C=C bonds, whereas the most intense bands between 1100 and 1000 cm−1 correspond to C–O bonds, which indicated there were alcohols and phenols in the samples.
FTIR spectrum confirmed the presence of bioactive compounds in hawthorn berries extracts. These bioactive compounds were presumed to act as reducing and capping agents for AgNPs and CuNPs. The presence of functional groups such as C–O, C=C and C=O derived from alkaloids, flavones and anthracenes helped in the synthesis of metal nanoparticles (Neagu et al. 2013).
Antimicrobial activity of synthesised metal nanoparticles
The growth of the microorganisms treated with the prepared nanoparticles suspension was assessed. A suspension of eight nanoparticles, differing in salts of silver or copper ions and a type of hawthorn extract, was chosen for the analysis. Table 4 presents the survival results of microorganisms. The results were presented as percent of survival compared to the reference samples (using water or ethanol instead of nanoparticle suspensions).
Based on the results of the carried out research, the increased microbiological activity of silver nanoparticles compared to copper nanoparticles was found. There was no significant effect of the type of extract on microbial activity of nanoparticles. All suspensions showed a high degree of inhibition of A. niger fungus after 24 h (Figs. 10, 11). In all systems, a decrease in the growth of microorganisms was observed compared to control samples by 2–34% on average for copper nanoparticles and by 9–79% for silver nanoparticles after 48 h. Both silver and copper nanoparticles showed the lowest activity against E. coli bacteria. The highest activity for both copper and silver nanoparticles was observed against S. cerevisiae, achieving a growth inhibition rate from 34 to 14% for copper and from 79 to 32% for silver compared to controls.
The basis of the mechanism of toxic action of metal nanoparticles is the high activity to release metal ions suitable for the nanoparticle (Cui et al. 2018). The particle size contained in the nanoscale allows them to migrate through membranes and cell walls, affecting cellular homeostasis (Shakeel et al. 2016). The limited microbiological activity of the obtained metal nanoparticles may result from the agglomeration of particles during and after the preparation process, which was confirmed by the SEM and STEM photomicrographs. Despite the narrow distribution of nanoparticles, the total particle size could not limit the growth of microorganisms. In the A. niger samples, delayed cell growth was observed. One of the main mechanisms of toxicity of nanoparticles is the formation of ROS, which can damage cell organelles and initiate the production of an increasing number of free radicals. ROS are able to oxidise double bonds of fatty acids in cell membranes, which results in increased permeability of membranes, contributing to osmotic stress (Thakur et al. 2018; Zain et al. 2014). It could cause inhibition of cell development. After 24 h, further agglomeration of nanoparticles occurred, which limited the negative effects of metal nanoparticles, causing an increase in the number of cells.
Conclusion
In this work, a green method for preparing silver and copper nanoparticles was developed using extract of hawthorn as a stabiliser and reductant. Water and ethanol extract of dried fruit of hawthorn have been reported for the reduction of silver and copper ions using organic and inorganic salts. Green synthesised metal nanoparticles showed roughly similar antibacterial activity against the selected pathogenic microorganisms (A. niger, E. coli and S. cerevisiae) at fairly low concentrations (below 100 mg/l). The metal nanoparticles, derived from inorganic salts, were monodisperse with spherical morphology with an average diameter of 60 and 200 nm for silver and copper nanoparticles, respectively. The presented green nanoparticles synthesis approach appears to be an alternative to the conventional physical and chemical synthesis methods.
References
Achuba FI (2018) Role of bitter leaf (Vernonia amygdalina) extract in prevention of renal toxicity induced by crude petroleum contaminated diets in rats. Int J Vet Sci Med 6(2):172–177
Alirezalu A, Salehi P, Ahmadi N, Sonboli A, Aceto S, Ayyari HHM (2018) Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int J Food Prop 21(1):452–470
Alkaya DB, Seyhan SA, Ozturk BN (2019) Influence of extraction method on antioxidant properties of Rheum ribes root extract. Ovidius Univ Ann Chem 30(1):44–47
Alves LA, Almeida JB, Giulietti M (2007) Solubility of d-glucose in water and ethanol/water mixtures. J Chem Eng Data 52:2166–2170
Anebi OP, Ugbe AF, Igwe CP, Odumu OF (2016) Determination of variation of vitamin “C” content of some fruits and vegetables consumed in Ugbokolo after prolonged storage. IOSR J Environ Sci 10(7):17–19
Baghizadeh A, Ranjbar S, Gupta VK, Asif M, Pourseyedi S, Karimi MJ, Mohammadinejad R (2015) Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J Mol Liq 207:159–163
Chang Q, Zuo Z, Harrison F, Chow MSS (2002) Hawthorn. J Clin Pharmacol 42(6):605–612
Chokkareddy R, Redhi GG (2018) Green metal nanoparticles: synthesis, characterization and their applications. Chapter IV Green synthesis of metal nanoparticles and its reaction mechanisms. Wiley, New York, pp 113–139
Cui D, Liang T, Sun L, Meng L, Yang C, Wang L, Liang T, Li Q (2018) Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm Biol 56(1):528–534
Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2011) Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv Nat Sci Nanosci Nanotechnol 2(1):015009
Della Pelle F, González MC, Sergi M, Del Carlo M, Compagnone D, Escarpa A (2015) Gold nanoparticles-based extraction-free colorimetric assay in organic media: an optical index for determination of total polyphenols in fat-rich samples. Anal Chem 87(13):6905–6911
Della Pelle F, Scroccarello A, Sergi M, Mascini M, Del Carlo M, Compagnone D (2018) Simple and rapid silver nanoparticles based antioxidant capacity assays: reactivity study for phenolic compounds. Food Chem 256:342–349
Della Pelle F, Scroccarello A, Scarano S, Compagnone D (2019) Silver nanoparticles-based plasmonic assay for the determination of sugar content in food matrices. Anal Chim Acta 1051:129–137
Din MI, Rehan R (2017) Synthesis, characterization, and applications of copper nanoparticles. Anal Lett 50(1):50–62
Fernández-Agulló A, Pereira E, Freire MS, Valentão P, Andrade PB, González-Álvarez J, Pereira JA (2013) Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 42:126–132
Fuleki T, Francis FJ (1968) Quantitative methods for anthocyanins. J Food Sci 33:266–274
Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38(4):545–560
Ibrahim HMM (2015) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 8(3):265–275
Jeevanandam J, Chan YS, Danquah MK (2016) Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev 3(2):55–67
Kang JP, Kim YJ, Singh P, Huo Y, Soshnikova V, Markus J, Ahn S, Chokkalingam M, Lee HA, Yang DC (2017) Biosynthesis of gold and silver chloride nanoparticles mediated by Crataegus pinnatifida fruit extract: in vitro study of anti-inflammatory activities. Artif Cells Nanomed Biotechnol 46(8):1530–1540
Khatoon UT, Mekala NK, Baadhe RR, Mohan MK, Bageswara Rai GVS (2015) Microbial inhibition of copper nanoparticles produced by chemical method. Nano Trends J Nanotechnol Appl 17(2):15–22
Kocira S, Szparaga A, Kocira A, Czerwińska E, Wójtowicz A, Bronowicka-Mielniczuk U, Koszel M, Findura P (2018) Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front Plant Sci 9:388
Kumar K (2017) Air pollution tolerance index of selected plants in Vijayawada city, Andhra Pradesh. Int J Green Pharm 11(4):877–881
Kumar KP, Reddy VR, Prakash MG, Kumar PK (2018) In vitro estimation of total phenolics and DPPH radical scavenging activity of Withania somnifera extract. Pharma Innov J 7(3):588–590
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—an updated report. Saudi Pharm J 24(4):473–484
Mercado N, Bhatt P, Sutariya V, Florez FLE, Pathak YV (2019) Application of Nanoparticles in treating periodontitis: preclinical and clinical overview. Surface modification of nanoparticles for targeted drug delivery, application of nanoparticles in treating periodontitis: preclinical and clinical overview. Springer, pp 467–480
Miao J, Li X, Fan Y, Zhao C, Mao X, Chen X, Huang H, Gao W (2016) Effect of different solvents on the chemical composition, antioxidant activity and alpha-glucosidase inhibitory activity of hawthorn extracts. Int J Food Sci Technol 51(5):1244–1251
Mittal A, Chisti Y, Banerjee U (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31(2):346–356
Neagu M, Pascu DE, Traistaru GA, Cretu G, Nechifor AC, Bunaciu AA, Aboul-Enein Y (2013) Membrane separation and concentration study of biological active compounds. Anal Chem Lett 3(5–6):314–321
Öztürk N, Tunçel M (2011) Assessment of phenolic acid content and in vitro antiradical characteristics of Hawthorn. J Med Food 14(6):664–669
Padma PN, Kumari SC, Banu ST (2018) Effect of diverse factors on green synthesis of copper nanoparticles. Int J Life Sci 6(2):659–664
Pandurangan P, Sahadeven M, Sunkar S, Mohana Dhana SKN (2018) Comparative analysis of biochemical compounds of leaf, flower and fruit of Couroupita guianensis and synthesis of silver nanoparticles. Pharmacogn J 10(2):315–323
Pasandide B, Khodaiyan F, Mousavi Z, Hosseini SS (2017) Pectin extraction from citron peel: optimization by Box–Behnken response surface design. Food Sci Biotechnol 27(4):997–1005
Pelle Della F, Compagnone D (2018) Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 18(2):462
Salmanian S, Sadeghi Mahoonak AR, AlAmi M, Gorbani M (2014) Evaluation of total phenolic, flavonoid, anthocyanin compounds, antibacterial and antioxidant activity of hawthorn (Crataegus elbursensis) fruit acetonic extract. J Rafsanjan Univ Med Sci 13(1):53–66
Shaheen MNF, El-hadedy DE, Ali ZI (2019) Medical and microbial applications of controlled shape of silver nanoparticles prepared by ionizing radiation. BioNanoScience 9(2):414–422
Shakeel A, Mudasir A, Babu LS, Saiqa I (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28
Shrivas K, Sahu S, Kumar Patra G, Kumar Jaiswal N, Shankar R (2016) Localized surface plasmon resonance of silver nanoparticles for sensitive colorimetric detection of chromium in surface water, industrial waste water and vegetable samples. Anal Methods 8:2088–2096
Tadić VM, Dobrić S, Marković GM, Ðorđević SM, Arsić IA, Menković NR, Stević T (2008) Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem 56(17):7700–7709
Thakur S, Sharma S, Thakur S, Rai R (2018) Green synthesis of copper nano-particles using Asparagus adscendens Roxb root and leaf extract and their antimicrobial activities. Int J Curr Microbiol Appl Sci 7(4):683–694
Urbinaviciǔte A, Jakstas V, Kornysova O, Janulis V, Maruska A (2006) Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. J Chromatogr A 1112(1–2):339–344
Yasir M, Singh J, Tripathi MK, Singh P, Shrivastava R (2018) Green synthesis of silver nanoparticles using leaf extract of common arrowhead houseplant and its anticandidal activity. Pharmacogn Mag 13(4):840–844
Young-Tae K, Gu-Dam L, Seil KS, Han R, Hyo-Ryoung L, Yong-Ho C (2019) Effect of localized surface plasmon resonance on dispersion stability of copper sulfide nanoparticles. Appl Surf Sci 477:204–210
Zain NM, Stapley AGF, Shama G (2014) Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohyd Polym 112:195–202
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Długosz, O., Chwastowski, J. & Banach, M. Hawthorn berries extract for the green synthesis of copper and silver nanoparticles. Chem. Pap. 74, 239–252 (2020). https://doi.org/10.1007/s11696-019-00873-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00873-z