Abstract
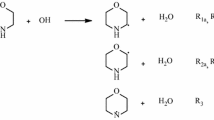
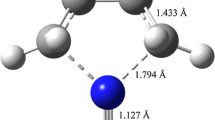
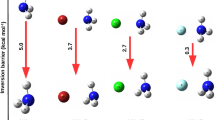
The effects of chemical modification on the kinetics of GSPT in 6R2PY-NH3 (R=NO2, CF3, COOH; CH3, C2H5) complex, in which the H atom at C6 position of 2PY was substituted, were researched in detail at the M06-2X/6-311 + G(d, p) level. The changes of structural parameter, reaction mechanism, and energies of GSPT before and after the replacement with different substituent have been analyzed. GSPT process in the 6R2PY-NH3 (R=NO2, CF3, COOH; CH3, C2H5) complex preferred to occur via a concerted but asynchronous protolysis pathway regardless of the electronic nature of substituent R. However, the structural parameters, asynchronicity of GSPT, and barrier height were influenced by the different substituent. The Hammett’s and Taft’s substituent constant had linear correlation with ∆(R1 + R2), NBO charges of NH4+ and ∆∆V.








Similar content being viewed by others
References
Blaser S, Ottiger P, Lobsiger S et al (2011) Intermolecular clamping by hydrogen bonds: 2-pyridone-NH3. Chem Phys Chem 12:1841–1850. https://doi.org/10.1002/cphc.201100037
Borst DR, Roscioli JR, Pratt DW et al (2002) Hydrogen bonding and tunneling in the 2-pyridone.2-hydroxypyridine dimer. Effect of electronic excitation. Chem Phys 283:341–354. https://doi.org/10.1016/S0301-0104(02)00598-0
Brause R, Schmitt M, Kleinermanns K (2007) Improved determination of structural changes of 2-pyridone-(H2O)1 upon electronic excitation. J Phys Chem A 111:3287–3293. https://doi.org/10.1021/jp067572b
Brown ID (1992) Chemical and steric constraints in inorganic solids. Acta Cryst B 48:553–572. https://doi.org/10.1107/S0108768192002453
Carney JR, Fedorov AV, Cable JR et al (2001) Infrared spectroscopy of H-bonded bridges stretched across the cis-amide group: I. water bridges. J Phys Chem A 105:3487–3497. https://doi.org/10.1021/jp003375f
Chen YL, Wu DY, Tian ZQ (2016) Theoretical investigation on the substituent effect of halogen atoms at the C8 position of adenine: relative stability, vibrational frequencies, and raman of tautomers. J Phys Chem A 120:4049–4058. https://doi.org/10.1021/acs.jpca.6b03604
Esboui M, Nsangou M, Jaidane N et al (2005) DFT study of ground state proton transfer in 2-pyridone/hydroxypyridine-ammonia clusters. Chem Phys 311:277–285. https://doi.org/10.1016/j.chemphys.2004.11.022
Fang WH (1998) Ab initio study of the triple-proton-transfer reactions of ground and excited states of 7-hydroxyquinoline in methanol solution. J Am Chem Soc 120:7568–7576. https://doi.org/10.1021/ja973710m
Fang WH (1999) Theoretical characterization of the structures and reactivity of 7-hydroxyquinoline-(H2O)n (n = 1–3) complexes. J Am Chem Soc 103:5567–5573. https://doi.org/10.1021/jp990524p
Faxen K, Gilderson G, Adelroth P et al (2005) A mechanistic principle for proton pumping by cytochrome c oxidase. Nature 437:286–289. https://doi.org/10.1038/nature03921
Florio GM, Gruenloh CJ, Quimpo RC et al (2000) The infrared spectroscopy of hydrogen-bonded bridges: 2-pyridone-(water)n and 2-hydroxypyridine-(water)n clusters, n = 1, 2. J Chem Phys 113:11143–11153. https://doi.org/10.1063/1.1324613
Frisch MJ et al (2009) Gaussian 09. Gaussian Inc, Wallingford
Glendening ED (2005) Natural energy decomposition analysis: extension to density functional methods and analysis of cooperative effects in water clusters. J Phys Chem A 109:11936–11940. https://doi.org/10.1021/jp058209s
Glendening ED, Landis CR, Weinhold F (2013) NBO 6.0: natural bond orbital analysis program. J Comput Chem 35:1429–1437. https://doi.org/10.1002/jcc.23266
Hansch C, Leo A, Taft RW (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91:165–195. https://doi.org/10.1021/cr00002a004
Hay S, Pudney CR, McGrory TA et al (2009) Barrier compression enhances an enzymatic hydrogen-transfer reaction. Angew Chem Int Ed 48:1452–1454. https://doi.org/10.1002/anie.200805502
Held A, Pratt D (1990) The 2-pyridone dimer, a model cis-peptide. Gas-phase structure from high-resolution laser spectroscopy. J Am Chem Soc 112:8629–8630. https://doi.org/10.1021/ja00179a083
Held A, Pratt D (1992) Hydrogen bonding in the symmetry-equivalent C2h dimer of 2-Pyridone in its S0 and S2 states: effect of deuterium substitution. J Chem Phys 96:4869–4876. https://doi.org/10.1063/1.462777
Held A, Pratt D (1993a) Hydrogen-bonding in water complexes-structures of 2-Pyridone-H2O and 2-Pyridone-(H2O)2 in Their S0 and S1 States. J Am Chem Soc 115:9708–9717. https://doi.org/10.1021/ja00074a042
Held A, Pratt D (1993b) Ammonia as a hydrogen-bond donor and acceptor in the gas-phase structures of 2-Pyridone-NH3 and 2-Pyridone-(NH3)2 in their S0 and S1 electronic states. J Am Chem Soc 115:9718–9723. https://doi.org/10.1021/ja00074a043
Held A, Champagne BB, Pratt D (1991) Inertial axis reorientation in the S1 ← S0 electronic transition of 2-Pyridone. A rotational Duschinsky effect. Structural and dynamic consequences. J Chem Phys 95:8732–8743. https://doi.org/10.1063/1.461209
Hung VP, Robert SP, Audrey GR et al (2014) Intramolecular Diels-Alder reactions of cycloalkenones: stereoselectivity, lewis acid acceleration, and halogen substituent effects. J Am Chem Soc 136:2397–2403. https://doi.org/10.1021/ja410220w
Kohen A, Cannio R, Bartolucci S et al (1999) Enzyme dynamics and hydrogen tunneling in a thermophilic alcohol dehydrogenase. Nature 399:496–499. https://doi.org/10.1038/20981
Li Q, Fang W, Yu J (2005) Theoretical studies of proton-transfer reactions of 2-hydroxypyridine-(H2O)n (n = 0–2) in the ground and excited states. J Phys Chem A 109:3983–3990. https://doi.org/10.1021/jp044498t
Lill MA, Helms V (2002) Proton shuttle in green fluorescent protein studied by dynamics simulations. Proc Natl Acad Sci 99:2778–2781. https://doi.org/10.1073/pnas.052520799
Limbach HH (2007) Hydrogen-Transfer Reactions. Schowen RW, Klinman JP, Hynes JT, Limbach HH (eds.). Wiley, Weinheim, Chapter 6, pp. 135-221
Limbach HH, Pietrzak M, Benedict H et al (2004) Empirical corrections for anharmonic zero-point vibrations of hydrogen and deuterium in geometric hydrogen bond correlations. J Mol Struct 706:115–119. https://doi.org/10.1016/j.molstruc.2004.03.006
Limbach HH, Lopez JM, Kohen A (2006) Arrhenius curves of hydrogen transfers: tunnel effects, isotope effects and effects of pre-equilibria. Philos Trans R Soc B 361:1399–1451. https://doi.org/10.1098/rstb.2006.1872
Limbach HH, Schowen KB, Schowen RL (2010) Heavy atom motions and tunneling in hydrogen transfer reactions: the importance of the pre-tunneling state. J Phys Org Chem 23:586–605. https://doi.org/10.1002/poc.1663
Lu D, Voth GA (1998) Proton transfer in the enzyme carbonic anhydrase: an ab initio study. J Am Chem Soc 120:4006–4014. https://doi.org/10.1021/ja973397o
Mathias G, Marx D (2007) Structures and spectral signatures of protonated water networks in bacteriorhodopsin. Proc Natl Acad Sci 104:6980–6985. https://doi.org/10.1073/pnas.0609229104
Matsuda Y, Ebata T, Mikami N (1999) Vibrational spectroscopy of 2-Pyridone and its clusters in supersonic jets: structures of the clusters as revealed by characteristic shifts of the N–H and C=O bands. J Chem Phys 110:8397–8407. https://doi.org/10.1063/1.478748
Matsuda Y, Ebata T, Mikami N (2000) Population labeling spectroscopy for the electronic and the vibrational transitions of 2-Pyridone and its hydrogen-bonded clusters. J Chem Phys 113:573–580. https://doi.org/10.1063/1.481833
Matsuda Y, Ebata T, Mikami N (2001) IR-UV double resonance spectroscopic study of 2-hydroxypyridine and its hydrogen-bonded clusters. J Phys Chem A 105:3475–3480. https://doi.org/10.1021/jp003272x
Mohammed OF, Pines D, Nibbering ETJ et al (2007) Base-induced solvent switches in acid-base reactions. Angew Chem Int Ed 46:1458–1461. https://doi.org/10.1002/anie.200603383
Müller A, Talbot F, Leutwyler S (2000) Intermolecular vibrations of jet-cooled (2-Pyridone)2: a model for the uracil dimer. J Chem Phys 112:3717–3725. https://doi.org/10.1063/1.480524
Müller A, Talbot F, Leutwyler S (2002) S1/S2 exciton splitting in the (2-Pyridone)2 dimer. J Chem Phys 116:2836–2847. https://doi.org/10.1063/1.1434987
Müller A, Losada M, Leutwyler S (2004) Ab initio benchmark study of (2-Pyridone)2, a strongly bound doubly hydrogen bonded dimer. J Phys Chem A 108:157–165. https://doi.org/10.1021/jp0361024
Ni M, Fang H (2019) Chem Select 4:1316–1322. https://doi.org/10.1002/slct.201802647
Nimlos MR, Kelley DF, Bernstein ER (1989) Spectroscopy, structure and proton dynamics of 2-hydroxypyridine and its clusters with water and ammonia. J Phys Chem 93:643–651. https://doi.org/10.1021/j100339a030
Sakota K, Tokuhara S, Sekiya H (2007) Dispersed fluorescence spectroscopy of 2-hydroxypyridine and its cyclically hydrogen-bonded water clusters in the gas phase: an examination of occurrence of excited-state proton transfer. Chem Phys Lett 448:159–163. https://doi.org/10.1016/j.cplett.2007.09.085
Tuckerman ME, Marx D, Parrinello M (2002) The nature and transport mechanism of hydrated hydroxide ions in aqueous solution. Nature 417:925–929. https://doi.org/10.1038/nature00797
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. University Press, Cambridge
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited-states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 21403114) and the Natural Science Foundation of Jiangsu province (No. BK20140970).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ni, M., Fang, H. Theoretical study on kinetics of ammonia-catalyzed ground-state tautomerization in 2-pyridone: effect of chemical modification. Chem. Pap. 73, 1561–1569 (2019). https://doi.org/10.1007/s11696-019-00711-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00711-2