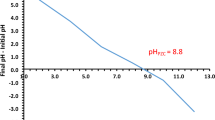
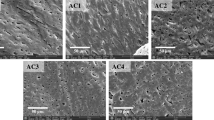
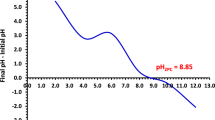
Abstract
The authors explore the impact of acid treatments on the N-doped porous carbon obtained from fish scales and its Cr(VI) removal capability. HCl and HNO3 are employed to treat the materials to remove template. The materials were characterized by elemental analysis, N2 adsorption/desorption isotherms, X-ray photoelectron spectroscopy and so on. Compared with the materials treated with HNO3, the materials treated with HCl have the larger specific surface area leading to the much higher adsorption capacity and faster adsorption rate of Cr(VI). To investigate the adsorption mechanism, the materials after adsorption of Cr(VI) were measured by X-ray photoelectron spectroscopy. It is found that the adsorbed Cr(VI) is partly reduced to Cr(III). The as-produced Cr(III) is in proportion to the content of quaternary-N in the carbons, which demonstrates the redox reaction between the N-doped porous carbon and Cr(VI). Remarkably, the materials carbonized at 800 °C treated with HCl reach equilibrium in 100 min and achieve the maximum capacity up to 416.67 mg g−1.








Similar content being viewed by others
References
Beukes JP, van Zyl PG, Ras M (2012) Treatment of Cr(VI)-containing wastes in the South African ferrochrome industry-a review of currently applied methods. J South Afr Inst Min Metall 112:347–352
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash–a sugar industry waste. J Colloid Interface Sci 271:321–328. doi:10.1016/j.jcis.2003.11.007
He J, Long Y, Wang Y, Wei C, Zhan J (2016) Aerosol-assisted self-assembly of reticulated N-doped carbonaceous submicron spheres for effective removal of hexavalent chromium. ACS Appl Mater Interfaces 8:16699–16707. doi:10.1021/acsami.6b04292
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340. doi:10.1205/095758298529696
Huang B, Shao H, Liu N, Xu ZJ, Huang Y (2015) From fish scales to highly porous N-doped carbon: a low cost material solution for CO2 capture. RSC Adv 5:88171–88175. doi:10.1039/c5ra16745k
Ihsanullah, Al-Khaldi FA, Abu-Sharkh B, Abulkibash AM, Qureshi MI, Laoui T, Atieh MA (2015) Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalination Water Treat 57:7232–7244. doi:10.1080/19443994.2015.1021847
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium (VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124:192–199
Kim SD, Park KS, Gu MB (2002) Toxicity of hexavalent chromium to Daphnia magna: influence of reduction reaction by ferrous iron. J Hazard Mater 93:155–164. doi:10.1016/S0304-3894(02)00057-2
Kyzas GZ, Matis KA (2015) Nanoadsorbents for pollutants removal: a review. J Mol Liq 203:159–168. doi:10.1016/j.molliq.2015.01.004
Li Y et al (2013) N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Res 47:4188–4197. doi:10.1016/j.watres.2012.10.056
Li C, Huang B, Li C, Chen X, Huang Y (2016a) In situ formation of nanoscale zero-value iron on fish-scale-based porous carbon for Cr(VI) adsorption. Water Sci Technol 73:2237–2243. doi:10.2166/wst.2016.077
Li M, Gong Y, Lyu A, Liu Y, Zhang H (2016b) The applications of populus fiber in removal of Cr(VI) from aqueous solution. Appl Surf Sci 383:133–141. doi:10.1016/j.apsusc.2016.04.167
Lian F, Cui G, Liu Z, Duo L, Zhang G, Xing B (2016) One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J Environ Manage 176:61–68. doi:10.1016/j.jenvman.2016.03.043
Liu Z, Du Z, Xing W, Yan Z (2014) Facial synthesis of N-doped microporous carbon derived from urea furfural resin with high CO2 capture capacity. Mater Lett 117:273–275. doi:10.1016/j.matlet.2013.12.021
Qi J, Chen S, Cui X, Hu T, Shen W (2014) High surface area nitrogen-containing porous carbon synthesis and adsorption of Pb(II) and Cr(VI) ions. Sci Adv Mater 6:963–969. doi:10.1166/sam.2014.1860
Rangabhashiyam S, Selvaraju N (2015) Efficacy of unmodified and chemically modified Swietenia mahagoni shells for the removal of hexavalent chromium from simulated wastewater. J Mol Liq 209:487–497. doi:10.1016/j.molliq.2015.06.033
Robin Wilson G, Sharma A, Sachdev D, Dubey A (2016) Ferulic acid functionalized mesoporous silica polymer nanocomposites (SBA/FA) for the adsorption of Cr(VI). J Porous Mater 23:195–200. doi:10.1007/s10934-015-0070-2
Sevilla M, Valle-Vigón P, Fuertes AB (2011) N-doped polypyrrole-based porous carbons for CO2 capture. Adv Func Mater 21:2781–2787. doi:10.1002/adfm.201100291
Shariati S, Khabazipour M, Safa F (2017) Synthesis and application of amine functionalized silica mesoporous magnetite nanoparticles for removal of chromium(VI) from aqueous solutions. J Porous Mater 24:129–139. doi:10.1007/s10934-016-0245-5
Sing KSW (1985) Reporting physosorption data for gas/solid systems with special reference to the determination of surface area and porosity. IUPAC Pure Appl Chem 57:603–619
Sun X, Yang L, Li Q, Zhao J, Li X, Wang X, Liu H (2014) Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chem Eng J 241:175–183. doi:10.1016/j.cej.2013.12.051
Wei H, Zhang Q, Zhang Y, Yang Z, Zhu A, Dionysiou DD (2016) Enhancement of the Cr(VI) adsorption and photocatalytic reduction activity of g-C3N4 by hydrothermal treatment in HNO3 aqueous solution. Appl Catal A 521:9–18. doi:10.1016/j.apcata.2015.11.005
Zhang S, Wang X, Li J, Wen T, Xu J, Wang X (2014) Efficient removal of a typical dye and Cr(VI) reduction using N-doped magnetic porous carbon. RSC Adv 4:63110–63117. doi:10.1039/c4ra10189h
Zhang X, Lin D, Chen W (2015) Nitrogen-doped porous carbon prepared from a liquid carbon precursor for CO2 adsorption. RSC Adv 5:45136–45143. doi:10.1039/c5ra08014b
Acknowledgements
Financial support from the National Natural Science Foundation of China (Nos. 51272017 and 51432003) is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, F., Wang, M., Huang, B. et al. Acid-treatment effect on the N-doped porous carbon obtained from fish scales for Cr(VI) removal. Chem. Pap. 71, 2261–2269 (2017). https://doi.org/10.1007/s11696-017-0220-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0220-x