Abstract
Background
Obesity is a worldwide epidemic leading to non-alcoholic fatty liver disease. Alterations in the liver fat fraction (LFF) assessed by MRI following bariatric surgery is a promising feature; however, few studies have been fully elucidated.
Purpose
To determine the alterations in the LFF features following surgery using MRI, to determine the correlation with the clinical non-alcoholic steatohepatitis score (C-NASH score), and to identify the predictive factors for postoperative score changes.
Methods
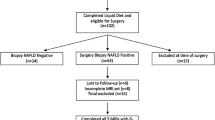
Patients (n = 69) underwent MRI to measure the LFF at baseline and 3 months postoperatively. Paired sample t tests were applied to investigate the alterations in the major parameters. Univariate analyses were performed to evaluate the factors predicting C-NASH score changes after surgery.
Results
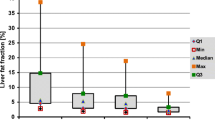
Compared with the baseline levels, the LFF significantly decreased 3 months postoperatively (P < 0.001). Significant positive correlations were detected between the C-NASH score and LFF levels (P < 0.001). Among the ROC curves for C-NASH score change, the AUC for the ROC curve of LFF was 0.812 (95% CI 0.707, 0.916) and the cut-off value was 6.16%. Weight at baseline was a significant predictive factor for postoperative changes when the C-NASH score was ≥ 3 (P < 0.001). The AUC for the ROC curve of weight was 0.897 (95% CI 0.782, 1.000) and 117 kg was the cut-off value.
Conclusions
LFF decreased following bariatric surgery, which predicted C-NASH score changes after surgery. For patients with a higher risk of NASH (score ≥ 3) at baseline and lower preoperative body weight, we noted significantly greater effects of surgery on score change value.





Similar content being viewed by others
References
Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100(6):2231–8.
Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51(3):433–45.
Reeder SB et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34(4):spcone.
Noworolski SM, Lam MM, Merriman RB, et al. Liver steatosis: concordance of MR imaging and MR spectroscopic data with histologic grade. Radiology. 2012;264(1):88–96.
Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267(3):767–75.
Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422–31.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Tai CM et al. Derivation and validation of a scoring system for predicting nonalcoholic steatohepatitis in Taiwanese patients with severe obesity. Surg Obes Relat Dis. 2017;13(4):686–92.
Hedderich DM, Hasenberg T, Haneder S, et al. Effects of bariatric surgery on non-alcoholic fatty liver disease: magnetic resonance imaging is an effective, non-invasive method to evaluate changes in the liver fat fraction. Obes Surg. 2017;27(7):1755–62.
Pooler BD, Wiens CN, McMillan A, et al. Monitoring fatty liver disease with MRI following bariatric surgery: a prospective, dual-center study. Radiology. 2019;290(3):682–90.
Steven S, Hollingsworth KG, Small PK, et al. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in type 2 diabetes. Diabet Med. 2016;33(12):1723–31.
Jorgensen NB et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9.
Dirksen C, Bojsen-Møller KN, Jørgensen NB, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia. 2013;56(12):2679–87.
Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31(9):1285–97.
Kim YO, Schuppan D. When GLP-1 hits the liver: a novel approach for insulin resistance and NASH. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G759–61.
Sharma S, Mells JE, Fu PP, et al. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6(9):e25269.
Bojsen-Moller KN et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63(5):1725–37.
Jorgensen NB et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–31.
Lassailly G, Caïazzo R, Pattou F, et al. Bariatric surgery for curing NASH in the morbidly obese? J Hepatol. 2013;58(6):1249–51.
Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500.
Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–906.
Gholam PM, Flancbaum L, Machan JT, et al. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102(2):399–408.
Campos GM, Bambha K, Vittinghoff E, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47(6):1916–23.
Anty R, Iannelli A, Patouraux S, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32(11–12):1315–22.
Ulitsky A, Ananthakrishnan AN, Komorowski R, et al. A noninvasive clinical scoring model predicts risk of nonalcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2010;20(6):685–91.
Pulzi FB, Cisternas R, Melo MR, et al. New clinical score to diagnose nonalcoholic steatohepatitis in obese patients. Diabetol Metab Syndr. 2011;3(1):3.
Tan CH, al-Kalifah N, Ser KH, et al. Long-term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): an external validation and application of a clinical NASH score. Surg Obes Relat Dis. 2018;14(10):1600–6.
Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274(2):416–25.
Permutt Z, le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36(1):22–9.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Garg H, Aggarwal S, Shalimar, et al. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg Obes Relat Dis. 2018;14(1):81–91.
Caiazzo R et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260(5):893–8. discussion 898-9
Praveen Raj P, Gomes RM, Kumar S, et al. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: "NASHOST" prospective observational trial. Surg Obes Relat Dis. 2015;11(6):1315–22.
Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16(10):1278–86.
Stratopoulos C, Papakonstantinou A, Terzis I, et al. Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes Surg. 2005;15(8):1154–60.
O’Kane M, Parretti HM, Hughes CA, et al. Guidelines for the follow-up of patients undergoing bariatric surgery. Clin Obes. 2016;6(3):210–24.
Acknowledgments
The authors would like to thank all of the involved study investigators, staffs, clinicians, nurses, and technicians for dedicating their time and skills to the completion of this study.
Funding
This study was supported by National Key Technologies R&D Program (Grant No. 2015BAI13B09), National Key Technologies R&D Program of China (No. 2017YFC0110904), Research Foundation of Beijing Friendship Hospital, Capital Medical University (Grant No. yyqdkt 2017-31), and Beijing Municipal Administration of Hospitals Incubating Program (Grant No. PX2018001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in this study.
Statement of Human and Animal Rights
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committees of Beijing Friendship Hospital, Capital Medical University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Cao, D., Liu, Y. et al. Alterations in the Liver Fat Fraction Features Examined by Magnetic Resonance Imaging Following Bariatric Surgery: a Self-Controlled Observational Study. OBES SURG 30, 1917–1928 (2020). https://doi.org/10.1007/s11695-020-04415-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04415-5