Abstract
Background
Vagal nerve stimulation (VNS) has been reported to reduce body weight and improve sympathovagal imbalance in both basic and clinical studies. Its effects on glycemic control were however unclear. The aims of this study were to investigate the effects of VNS with various parameters on blood glucose and its possible mechanisms in rats.
Methods
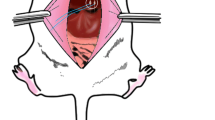
A hyperglycemic rodent model induced by glucagon was used initially to optimize the VNS parameters; then, a type 2 diabetic rodent model induced by high-fat diet combined with streptozotocin was used to validate the VNS method. The VNS electrodes were implanted at the dorsal subdiaphragmatic vagus; three subcutaneous electrodes were implanted at the chest area for recording electrocardiogram in rats induced by glucagon.
Results
(1) VNS with short pulse width of 0.3 ms but not 3 ms reduced blood glucose during an oral glucose tolerance test (OGTT), with a 38.4% reduction at 15 min and 26.9% at 30 min (P < 0.05, vs. sham-VNS respectively). (2) VNS at low frequency of 5 Hz but not 14 Hz or 40 Hz reduced blood glucose during the OGTT (P < 0.05, vs. sham-VNS). (3) Intermittent VNS was more potent than continuous VNS (P < 0.01). (4) No difference was found between unilateral VNS and bilateral VNS. (5) VNS enhanced vagal activity (P = 0.005). (6) The hypoglycemic effect of VNS was blocked by glucagon-like peptide-1 (GLP-1) antagonist exendin-4.
Conclusions
VNS at 5 Hz reduces blood glucose in diabetic rats by enhancing vagal efferent activity and the release of GLP-1.






Similar content being viewed by others
References
Introduction: standards of medical care in diabetes-2018. Diabetes Care. 2018 Jan;41(Suppl 1):S1-S2. Epub 2017/12/10.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28. https://doi.org/10.2337/dci18-0007.
Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metrics. 2010;8:29.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–36.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30.
Zimmet P, Alberti KG. Surgery or medical therapy for obese patients with type 2 diabetes? N Engl J Med. 2012;366(17):1635–6.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85.
Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016 Jan;39 Suppl 1:S4–5. Epub 2015/12/24.
Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–82.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–815.
Grimonprez A, Raedt R, Baeken C, et al. The antidepressant mechanism of action of vagus nerve stimulation: evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34.
Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276–86.
Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713–28.
Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9.
Bugajski AJ, Gil K, Ziomber A, et al. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J Physiol Pharmacol. 2007;58(Suppl 1):5–12.
Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes. 2007;31(11):1756–9.
Sobocki J, Fourtanier G, Estany J, et al. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006;139(2):209–16.
Ahren B, Taborsky Jr GJ. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118(4):1551–7.
Li S, Zhai X, Rong P, et al. Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One. 2014;9(11):e112066.
Malbert CH, Picq C, Divoux JL, et al. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes. 2017;66(4):848–57.
Ouyang X, Li S, Foreman R, et al. Hyperglycemia-induced small intestinal dysrhythmias attributed to sympathovagal imbalance in normal and diabetic rats. Neurogastroenterol Motil. 2015;27(3):406–15.
Ye F, Liu Y, Li S, et al. Hypoglycemic effects of intestinal electrical stimulation by enhancing nutrient-stimulated secretion of GLP-1 in rats. Obes Surg. 2018;4
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
de Bem GF, Costa CA, Santos IB, et al. Antidiabetic effect of Euterpe oleracea Mart. (acai) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: a positive interaction. PLoS One. 2018;13(6):e0199207.
Wu W, Lin L, Lin Z, et al. Duodenum exclusion alone is sufficient to improve glucose metabolism in STZ-induced diabetes rats. Obes Surg. 2018;22
Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298(4):G563–70.
Bellahsene BE, Lind CD, Schirmer BD, et al. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Phys. 1992;262(5 Pt 1):G826–34.
Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1190–5.
Ionut V, Liu H, Mooradian V, et al. Novel canine models of obese prediabetes and mild type 2 diabetes. Am J Physiol Endocrinol Metab. 2010;298(1):E38–48.
Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312(9):915–22.
Shikora SA, Toouli J, Herrera MF, et al. Intermittent vagal nerve block for improvements in obesity, cardiovascular risk factors, and glycemic control in patients with type 2 diabetes mellitus: 2-year results of the VBLOC DM2 study. Obes Surg. 2016;26(5):1021–8.
Gil K, Bugajski A, Kurnik M, et al. Physiological and morphological effects of long-term vagal stimulation in diet induced obesity in rats. J Physiol Pharmacol. 2009;60(Suppl 3):61–6.
Gil K, Bugajski A, Thor P. Electrical vagus nerve stimulation decreases food consumption and weight gain in rats fed a high-fat diet. J Physiol Pharmacol. 2011;62(6):637–46.
Val-Laillet D, Biraben A, Randuineau G, et al. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55(2):245–52.
McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25(10):815–e636.
Yin J, Abell TD, McCallum RW, et al. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15(3):224–31. discussion 31
Chen JD, Qian L, Ouyang H, et al. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124(2):401–9.
Liu J, Qiao X, Chen JD. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49(5):729–37.
Chen JD, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol. 2017;11(5):407–18.
Song GQ, Zhu H, Lei Y, et al. Gastric electrical stimulation optimized to inhibit gastric motility reduces food intake in dogs. Obes Surg. 2015;25(6):1047–55.
Zhu H, Sallam H, Chen DD, et al. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Phys Regul Integr Comp Phys. 2007;293(5):R1875–81.
Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8(5):056013.
Morton JM, Shah SN, Wolfe BM, et al. Effect of vagal nerve blockade on moderate obesity with an obesity-related comorbid condition: the ReCharge study. Obes Surg. 2016;26(5):983–9.
Banni S, Carta G, Murru E, et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLoS One. 2012;7(9):e44813.
George MS, Sackeim HA, Rush AJ, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47(4):287–95.
Krieger JP, Arnold M, Pettersen KG, et al. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43.
Nakagawa A, Satake H, Nakabayashi H, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110(1):36–43.
Ronveaux CC, de Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav. 2014;135:222–9.
Funding
Jieyun Yin was partially supported by a grant from NIH-SPARC (Stimulating Peripheral Activity for Relieving Conditions) (1U18TR001926).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Statement of Animal Rights/Ethical Approval
All applicable institutional and /or national guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, J., Ji, F., Gharibani, P. et al. Vagal Nerve Stimulation for Glycemic Control in a Rodent Model of Type 2 Diabetes. OBES SURG 29, 2869–2877 (2019). https://doi.org/10.1007/s11695-019-03901-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03901-9