Abstract
Background
This study was carried out to investigate whether sex-related differences exist in the adipocyte expression of clock genes from subcutaneous abdominal and visceral fat depots in severely obese patients.
Methods
We investigated 16 morbidly obese patients, eight men and eight women (mean age 45 ± 20 years; mean BMI 46 ± 6 kg/m2), undergoing laparoscopic gastric bypass surgery. Biopsies were taken as paired samples [subcutaneous and visceral adipose tissue (AT)] at the beginning of the surgical process at 11:00 h in the morning. Metabolic syndrome features such as waist circumference, plasma glucose, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were also studied. The expression of clock genes (PER2, BMAL1, and CRY1) was measured by quantitative real-time PCR, Western blot, and immunohistochemical analysis.
Results
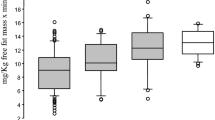
Gene expression was significantly higher in women than in men for the three genes studied in both ATs (P < 0.05). In visceral fat, these differences were more marked. (P < 0.001). Western blot analysis partially confirmed these results since statistical differences were observed for PER2 in both ATs and for CRY1 in subcutaneous adipose tissue. There were no differences in BMAL1 protein expression. Interestingly, clock gene expression level was correlated with LDL-C and HDL-C (P < 0.05). Moreover, we found significant associations with body fat mass in women and with age in men.
Conclusions
Clock genes expression is sex dependent in human adipose tissue from morbidly obese subjects and correlates to a decreased in metabolic syndrome-related traits. These preliminary results make necessary to go deep into the knowledge of the molecular basis of the sexual dimorphism in chronobiology.



Similar content being viewed by others
References
Garaulet M, Gómez-Abellán P, Madrid JA. Chronobiology and obesity: the orchestra out of tune. Clin Lipidol. 2010;5(2):181–8. Review.
Marcheva B, Ramsey KM, Affinati A, et al. Clock genes and metabolic disease. J Appl Physiol. 2009;107(5):1638–46. Review.
Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20(2):127–34.
Whitehead JP, Richards AA, Hickman IJ, et al. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80.
Gómez-Abellán P, Hernández-Morante JJ, Luján JA, et al. Clock genes are implicated in the human metabolic syndrome. Int J Obes. 2008;32(1):121–8.
Klaus S, Keijer J. Gene expression profiling of adipose tissue: individual, depot-dependent, and sex-dependent variabilities. Nutrition. 2004;20(1):115–20.
Garaulet M, Pérez-Llamas F, Fuente T, et al. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol. 2000;143(5):657–66.
Ramis JM, Salinas R, García-Sanz JM, et al. Depot- and gender-related differences in the lipolytic pathway of adipose tissue from severely obese patients. Cell Physiol Biochem. 2006;17(3–4):173–80.
Adan A, Natale V. Gender differences in morningness–eveningness preference. Chronobiol Int. 2002;19(4):709–20.
Lévi F, Filipski E, Iurisci I, et al. Cross-talks between circadian timing system and cell division cycle determine cancer biology and therapeutics. Cold Spring Harb Symp Quant Biol. 2007;72:465–75.
Mongrain V, Lavoie S, Selmaoui B, et al. Phase relationships between sleep–wake cycle and underlying circadian rhythms in morningness–eveningness. J Biol Rhythms. 2004;19(3):248–57.
Sopowski MJ, Hampton SM, Ribeiro DC, et al. Postprandial triacylglycerol responses in simulated night and day shift: gender differences. J Biol Rhythms. 2001;16(3):272–6.
Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20.
Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83.
Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–46.
Ando H, Yanagihara H, Hayashi Y, et al. Rhythmic mRNA expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146(12):5631–6.
Teboul M, Barrat-Petit MA, Li XM, et al. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med. 2005;83:693–9.
Sociedad Española para el Estudio de la Obesidad (SEEDO). Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica [article in Spanish]. Med Clin (Barc). 2007;128:184–96.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8.
Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80.
Yang X, Zhang YK, Esterly N, et al. Gender disparity of hepatic lipid homoeostasis regulated by the circadian clock. J Biochem. 2009;145(5):609–23.
Metz RP, Qu X, Laffin B, et al. Circadian clock and cell cycle gene expression in mouse mammary epithelial cells and in the developing mouse mammary gland. Dev Dyn. 2006;235:263–71.
Miller BH, Olson SL, Turek FW, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–73.
Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–6.
Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404.
Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology. 1978;102:1645–8.
Perrin JS, Segall LA, Harbour VL, et al. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci USA. 2006;103(14):5591–6.
Van Dongen HPA. Inter- and intra-individual differences in circadian phase. The Netherlands: Leiden University Press; 1998.
Wever RA. Characteristics of circadian rhythms in human functions. In Journal of Neural Transmission (Suppl.) Melatonin in Humans; Wurtman RJ, Waldhause F, Eds.; Springer, New York, 1986; 323–335.
Maywood ES, O'Brien JA, Hastings MH. Expression of mCLOCK and other circadian clock-relevant proteins in the mouse suprachiasmatic nuclei. J Neuroendocrinol. 2003;15(4):329–34.
Shende VR, Goldrick MM, Ramani S, et al. Expression and rhythmic modulation of circulating microRNAs targeting the clock gene Bmal1 in mice. PLoS One. 2011;6(7):e22586.
Lee J, Lee Y, Lee MJ, et al. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol. 2008;28(19):6056–65.
Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–69.
Saad MF, Riad-Gabriel MG, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83(2):453–9.
Bur IM, Cohen-Solal AM, Carmignac D, et al. The circadian clock components CRY1 and CRY2 are necessary to sustain sex dimorphism in mouse liver metabolism. J Biol Chem. 2009;284(14):9066–73.
Pilorz V, Steinlechner S, Oster H. Age and oestrus cycle-related changes in glucocorticoid excretion and wheel-running activity in female mice carrying mutations in the circadian clock genes Per1 and Per2. Physiol Behav. 2009;96(1):57–63.
Garaulet M, Corbalán MD, Madrid JA, et al. PER2 variants are associated with abdominal obesity, psycho-behavioral factors and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110(6):917–21.
Yang S, Liu A, Weidenhammer A, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythm. Endocrinology. 2009;150(5):2153–60.
Armitage R, Smith C, Thompson S, et al. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Res Online. 2001;4:33–41.
Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55.
Ando H, Ushijima K, Kumazaki M, et al. Influence of age on clock gene expression in peripheral blood cells of healthy women. J Gerontol A Biol Sci Med Sci. 2010;65(1):9–13.
Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21.
Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52.
Acknowledgments
This work was supported by the Government of Education, Science and Research of Murcia (Project BIO/FFA 07/01-0004) and by The Spanish Government of Science and Innovation (projects AGL2008-01655/ALI and AGL2008-04332/ALI and SAF2008-01432). CIBERehd is funded by the Instituto de Salud Carlos III, National Heart, Lung, and Blood Institute grants HL-54776, National Institute of Diabetes and Digestive and Kidney Diseases, grant number DK075030, and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research.
Disclosure statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Abellán, P., Madrid, J.A., Luján, J.A. et al. Sexual Dimorphism in Clock Genes Expression in Human Adipose Tissue. OBES SURG 22, 105–112 (2012). https://doi.org/10.1007/s11695-011-0539-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0539-2