Abstract
Background
The effects of surgical weight loss (WL) on inflammatory biomarkers associated with sleep apnea remain unknown. We sought to determine if any biomarkers can predict amelioration of sleep apnea achieved by bariatric surgery. We hypothesized that surgical WL would substantially reduce severity of sleep apnea and levels of proinflammatory cytokines.
Methods
Twenty-three morbidly obese adults underwent anthropometric measurements, polysomnography, and serum biomarker profiling prior to and 1 year following bariatric surgery. We examined the effect of WL and amelioration of sleep apnea on metabolic and inflammatory markers.
Results
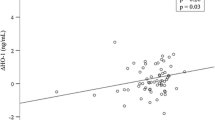
Surgical WL resulted in significant decreases in BMI (16.7 ± 5.97 kg/m2/median 365 days), apnea–hypopnea index (AHI), CRP, IL-6, sTNFαR1, sTNFαR2, and leptin levels, while ghrelin, adiponectin, and soluble leptin receptor concentrations increased significantly. Utilizing an AHI cutoff of 15 events/h, we found significantly elevated levels of baseline sTNFαR2 and greater post-WL sTNFαR2 decreases in subjects with baseline AHI ≥15 events/h compared to those with AHI <15 events/h despite no significant differences in baseline BMI, age, and ΔBMI. In a multivariable linear regression model adjusting for sex, age, impaired glucose metabolism, ΔBMI, and follow-up period, the post-WL decreases in AHI were an independent predictor of the decreases in sTNFαR2 and altogether accounted for 46% of the variance of ΔsTNFαR2 (P = 0.011) in the entire cohort.
Conclusions
Of all the biomarkers, the decrease in sTNFαR2 was independently determined by the amelioration of sleep apnea achieved by bariatric surgery. The results suggest that sTNFαR2 may be a specific sleep apnea biomarker across a wide range of body weight.




Similar content being viewed by others
References
McGinley BM, Schwartz AR, Schneider H, et al. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105:197–205.
Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol. 2007;92:51–65.
Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-α by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9.
Ciftci TU, Korturk O, Bukan N, et al. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91.
Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6.
Imagawa S, Yamaguchi Y, Ogawa K, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration. 2004;71:24–9.
Vgontzas AN, Zoumakis E, Bixler EO, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–95.
Kohler M, Ayers L, Pepperell JC, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax. 2009;64:67–73.
Schiza SE, Mermigkis C, Panagiotis P, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest. 2010;40:968–75.
Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. CPAP decreases plasma levels of soluble tumor necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32:1009–15.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42.
Renquist K. Obesity classification. Obes Surg. 1998;8:480.
Iber C, Ancoli-Israel S, Chesson A, et al for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed.: Westchester, Illinois: American Academy of Sleep Medicine, 2007.
American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89.
American Sleep Disorders Association. Practice parameters for the indications for polysomnography and related procedures: polysomnography task force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diab Care. 2003;26:3160–7.
Hohmann H-P, Brockhaus M, Bauerle PA, et al. Expression of the type A and B tumor necrosis factor (TNF) receptor is independently regulated, and both receptors mediate activation of the transcription factor NF-kB. J Biol Chem. 1990;265:22409–17.
Engelmann H, Novick D, Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990;265:1531–6.
Yue HJ, Mills PJ, Ancoli-Israel S, et al. The roles of TNF-alpha and the soluble TNF receptor I on sleep architecture in OSA. Sleep Breath. 2009;13:263–9.
Ronnemaa T, Pulkki K, Kaprio J. Serum soluble tumor necrosis factor-alpha receptor 2 is elevated in obesity but is not related to insulin sensitivity: a study in identical twins discordant for obesity. J Clin Endocrinol Metab. 2000;85:2728–32.
Benjafield AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med. 2001;79:109–15.
Bulló M, Garcia-Lorda P, Salas-Salvadó J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol. 2002;146:325–31.
Vanden Berghe W, Vermeulen L, De Wilde G, et al. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol. 2000;60:1185–95.
Ammit AJ, Lazaar AL, Irani C, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–74.
Bruun JM, Pedersen SB, Kristensen K, et al. Opposite regulation of interleukin-8 and tumor necrosis factor-alpha by weight loss. Obes Res. 2002;10:499–506.
Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–8.
Willi SM, Oexmann MJ, Wright NM, et al. The effects of a high-protein, low-fat, ketogenic diet on adolescents with morbid obesity: body composition, blood chemistries, and sleep abnormalities. Pediatrics. 1998;101:61–7.
Sinton CM, Fitch T, Gershenfeld HK. The effect of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research Funding Source
This study is supported by NIH HL 50381, HL80105, and NCRR UL1 RR 025005. Maria Pallayova is the recipient of a European Respiratory Society Fellowship Number LTRF 15-2008.
Rights and permissions
About this article
Cite this article
Pallayova, M., Steele, K.E., Magnuson, T.H. et al. Sleep Apnea Determines Soluble TNF-α Receptor 2 Response to Massive Weight Loss. OBES SURG 21, 1413–1423 (2011). https://doi.org/10.1007/s11695-011-0359-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0359-4