Abstract
Background
The obesity epidemic causes significant morbidity and mortality. Knowledge of cellular function and gene expression in obese adipose tissue will yield insights into obesity pathogenesis and suggest therapeutic targets. The aim of this work is to study the processes determining fat accumulation in adipose tissue from obese patients.
Methods
Omental fat was collected from two cohorts of obese bariatric surgery patients and sex-matched normal-weight donors. Isolated adipocytes were compared for cell size, volume, and long-chain fatty acid (LCFA) uptake. Omental fat RNAs were screened by 10K microarray (cohort 1: three obese, three normal) or Whole Genome microarray (cohort 2: seven obese, four normal). Statistical differences in gene and pathway expression were identified in cohort 1 using the GeneSifter Software (Geospiza) with key results confirmed in cohort 2 samples by microarray, quantitative real-time polymerase chain reaction, and pathway analysis.
Results
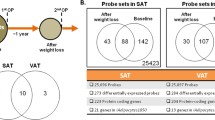
Obese omental adipocytes had increased surface area, volume, and V max for saturable LCFA uptake. Dodecenoyl-coenzyme A delta isomerase, central to LCFA metabolism, was approximately 1.6-fold underexpressed in obese fat in cohorts 1 and 2. Additionally, the Kyoto Encyclopedia of Genes and Genomics pathway analysis identified oxidative phosphorylation and fatty acid metabolism pathways as having coordinate, nonrandom downregulation of gene expression in both cohorts.
Conclusions
In obese omental fat, saturable adipocyte LCFA uptake was greater than in controls, and expression of key genes involved in lipolysis, β-oxidation, and metabolism of fatty acids was reduced. Thus, both increased uptake and reduced metabolism of LCFAs contribute to the accumulation of LCFAs in obese adipocytes.





Similar content being viewed by others
References
Stremmel W, Berk PD. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986;83(10):3086–90.
Schwieterman W, Sorrentino D, Potter BJ, et al. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988;85(2):359–63.
Sorrentino D, Stump D, Potter BJ, et al. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest. 1988;82(3):928–35.
Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest. 1988;82(6):2001–10.
Nunes RM IL, Sorrentino D, Berk PD. Oleate uptake by isolated hepatocytes consists of two components, each driven by the unbound oleate concentration. In: Proceedings of the 3rd International Congress, Mathematical Modelling of Liver Excretory Process, Juntendo University Press, Tokyo; 1990. p. 312–6.
Stump DD, Nunes RM, Sorrentino D, et al. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J Hepatol. 1992;16(3):304–15.
Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Mol Cell Biochem. 1999;192(1–2):17–31.
Stump DD, Fan X, Berk PD. Oleic acid uptake and binding by rat adipocytes define dual pathways for cellular fatty acid uptake. J Lipid Res. 2001;42(4):509–20.
Abumrad NA, Perkins RC, Park JH, et al. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981;256(17):9183–91.
Abumrad NA, Park JH, Park CR. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984;259(14):8945–53.
Glatz JF, van Nieuwenhoven FA, Luiken JJ, et al. Role of membrane-associated and cytoplasmic fatty acid-binding proteins in cellular fatty acid metabolism. Prostaglandins Leukot Essent Fatty Acids. 1997;57(4–5):373–8.
Luiken JJ, Turcotte LP, Bonen A. Protein-mediated palmitate uptake and expression of fatty acid transport proteins in heart giant vesicles. J Lipid Res. 1999;40(6):1007–16.
Luiken JJ, Glatz JF, Bonen A. Fatty acid transport proteins facilitate fatty acid uptake in skeletal muscle. Can J Appl Physiol. 2000;25(5):333–52.
Kampf JP, Kleinfeld AM. Fatty acid transport in adipocytes monitored by imaging intracellular free fatty acid levels. J Biol Chem. 2004;279(34):35775–80.
Kleinfeld AM, Kampf JP, Lechene C. Transport of 13C-oleate in adipocytes measured using multi imaging mass spectrometry. J Am Soc Mass Spectrom. 2004;15(11):1572–80.
Petrescu O, Fan X, Gentileschi P, et al. Long-chain fatty acid uptake is upregulated in omental adipocytes from patients undergoing bariatric surgery for obesity. Int J Obes (Lond). 2005;29(2):196–203.
Fan X, Bradbury MW, Berk PD. Leptin and insulin modulate nutrient partitioning and weight loss in ob/ob mice through regulation of long-chain fatty acid uptake by adipocytes. J Nutr. 2003;133(9):2707–15.
Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis. 2004;8(3):639–71. xi.
Berk PD, Zhou SL, Kiang CL, et al. Uptake of long chain free fatty acids is selectively up-regulated in adipocytes of Zucker rats with genetic obesity and non-insulin-dependent diabetes mellitus. J Biol Chem. 1997;272(13):8830–5.
Berk PD, Zhou SL, Bradbury M, et al. Regulated membrane transport of free fatty acids in adipocytes: role in obesity and non-insulin dependent diabetes mellitus. Trans Am Clin Climatol Assoc. 1997;108:26–40.
Berk PD, Zhou S, Kiang C, et al. Selective up-regulation of fatty acid uptake by adipocytes characterizes both genetic and diet-induced obesity in rodents. J Biol Chem. 1999;274(40):28626–31.
Berk PD. The master’s perspective: regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, & metabolic syndrome. Hepatology. 2008;48:1362–76.
Verna EC, Berk PD. Role of fatty acids in the pathogenesis of obesity and fatty liver: impact of bariatric surgery. Semin Liver Dis. 2008;28(4):407–26.
Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130(4):1343–6.
Di Girolamo M, Mendlinger S, Fertig JW. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971;221:850–8.
Wosilait WD, Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed. 1976;6:142–8.
Spector AA, Fletcher JE, Ashbrook JD. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971;10:3229–32.
Bojesen IN, Bojesen E. Binding of arachidonate and oleate to bovine serum albumin. J Lipid Res. 1994;35:770–8.
Richieri GV, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–80.
Rose H, Conventz M, Fischer Y, et al. Long-chain fatty acid-binding to albumin: re-evaluation with directly measured concentrations. Biochim Biophys Acta. 1994;1215:321–6.
Berman M, Weiss MF. Users’ manual for SAAM. Washington: US Government Printing Office; 1967.
SAAM II user guide. Seattle: SAAM Institute; 1998.
Sorrentino D, Robinson RB, Kiang CL, et al. At physiologic albumin/oleate concentrations oleate uptake by isolated hepatocytes, cardiac myocytes, and adipocytes is a saturable function of the unbound oleate concentration. Uptake kinetics are consistent with the conventional theory. J Clin Invest. 1989;84:1325–33.
Snedecor GW, Cochran WG. Statistical methods. 6th ed. Ames: Iowa State University Press; 1967.
Ramakrishnan R, Dorris D, Lublinsky A, et al. An assessment of Motorola CodeLink microarray performance for gene expression profiling applications. Nucleic Acids Res. 2002;30(7):e30.
Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9.
Kanehisa M, Goto S, Kawashima S, et al. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–80.
Doniger SW, Salomonis N, Dahlquist KD, et al. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4(1):R7.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes. 1987;11(2):129–40.
Edens NK, Fried SK, Kral JG, et al. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol. 1993;265(3 Pt 1):E374–9.
Ostman J, Arner P, Engfeldt P, et al. Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 1979;28(12):1198–205.
Russell CD, Petersen RN, Rao SP, et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res. 2002;34:650–4.
Chirieac DV, Chirieac LR, Corsetti JP, et al. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab. 2000;279(5):E1003–11.
Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33(2):138–44.
Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74.
Kampf JP, Parmley D, Kleinfeld AM. Free fatty acid transport across adipocytes is mediated by an unknown membrane protein pump. Am J Physiol Endocrinol Metab. 2007;293(5):E1207–14.
Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20(2):72–7.
Garaulet M, Pérez-Llamas F, Pérez-Ayala M, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–91.
Hsueh WC, Mitchell BD, Schneider JL, et al. Genomewide scan of obesity in the Old Order Amish. J Clin Endocrinol Metab. 2001;86:1199–205.
Freedman BI, Langefeld CD, Rich SS, et al. A genome scan for ESRD in black families enriched for nondiabetic nephropathy. J Am Soc Nephrol. 2004;15(10):2719–27.
Mustelin L, Pietiläinen KH, Rissanen A, et al. Acquired obesity and poor physical fitness impair expression of genes of mitochondrial oxidative phosphorylation in monozygotic twins discordant for obesity. Am J Physiol Endocrinol Metab. 2008;295(1):E148–54.
Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–71.
Kim K, Perroud B, Espinal G, et al. Genes and networks expressed in perioperative omental adipose tissue are correlated with weight loss from Roux-en-Y gastric bypass. Int J Obes. 2008;32:1395–406.
Gillingham MB, Purnell JQ, Jordan J, et al. Effects of higher dietary protein intake on energy balance and metabolic control in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2007;90(1):64–9.
Buijs MM, Burggraaf J, Wijbrandts C, et al. Blunted lipolytic response to fasting in abdominally obese women: evidence for involvement of hyposomatotropism. Am J Clin Nutr. 2003;77:544–50.
Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54(11):3190–7.
Shi L, Reid LH, Jones WD, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–61.
Bardag-Gorce F, Oliva J, Dedes J, et al. Chronic ethanol feeding alters hepatocyte memory which is not altered by acute feeding. Alcohol Clin Exp Res. 2009;33(4):684–92.
Trentin L, Giordan M, Dingermann T, et al. Two independent gene signatures in pediatric t(4;11) acute lymphoblastic leukemia patients. Eur J Haematol. 2009;in press.
Ukena SN, Koenecke C, Geffers R, et al. T helper type 2 differentiation is associated with induction of antibacterial defense mechanisms in blood lymphocytes of patients with sarcoidosis. Immunol Invest. 2009;38(1):49–66.
Kennedy L, Vass JK, Haggart DR, et al. Hematopoietic lineage transcriptome stability and representation in PAXgene collected peripheral blood utilising SPIA single-stranded cDNA probes for microarray. Biomarker Insights. 2008;3:403–17.
Acknowledgements
This study was supported by grants DK-52401 and DK-72526 from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health and by the Liver Disease Research Fund at Columbia University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walewski, J.L., Ge, F., Gagner, M. et al. Adipocyte Accumulation of Long-Chain Fatty Acids in Obesity is Multifactorial, Resulting from Increased Fatty Acid Uptake and Decreased Activity of Genes Involved in Fat Utilization. OBES SURG 20, 93–107 (2010). https://doi.org/10.1007/s11695-009-0002-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-0002-9