Background
Gastric/intestinal electrical stimulation (GIES) has been used to suppress appetite in the treatment of obesity with promising results. However, the mechanisms by which GIES benefits obese patients are not completely understood. This study investigated the acute effects of GIES on gastric and intestinal tissue levels of peptide hormones related to satiety and appetite in rats.
Methods
32 rats were divided into 4 groups: 1) sham stimulation, 2) gastric electrical stimulation (GES) with pulse trains, 3) GES with long pulse, and 4) duodenal electrical stimulation (DES) with pulse trains. After 2 hours of GIES, the rats were sacrificed immediately, and gastric fundus, duodenum and distal colon were harvested and extracted. Hormone levels of ghrelin, obestatin, cholecystokinin-8 (CCK-8) and peptide YY (PYY) were measured by radioimmunoassay (RIA).
Results
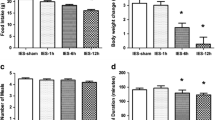
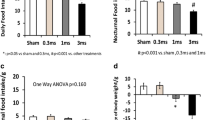
1) The mean gastric fundus ghrelin level was 1789.04 ± 362.81 pg/mg in the sham stimulation and significantly decreased with GES of pulse trains (597.85 ± 195.33 pg/mg, P = 0.012), GES of long pulse (754.6 ± 282.6 pg/mg, P = 0.039) and DES (731.69 ± 110.84pg/mg, P = 0.037). 2) The mean duodenal CCK-8 concentration was 413.27 ± 42.14 pg/mg in the sham stimulation and significantly increased by DES (762.6 ± 98.75 pg/mg, P = 0.013) but not in others. 3) Neither gastric obestatin nor distal colonic PYY was altered by any of GES or DES.
Conclusions
GIES significantly impacts appetiterelated peptide hormones in gastric and duodenal tissues. Acute GIES-induced manipulation of gut peptide hormones related to appetite and satiety is a nonpharmacologic, well-tolerated clinical procedure that could substantially contribute to the successful treatment and long-term management of obesity.
Similar content being viewed by others
References
Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood) 2001; 226: 963–77.
Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology 2005; 128: 175–91.
Small CJ, Bloom SR. Gut hormones as peripheral anti-obesity targets Current Drug Targets — CNS & Neurological Disorder 2004; 3: 379–88.
Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–13.
Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci 2001; 2: 551–60.
Kojima M, Kangawa K. Ghrelin, an orexigenic signaling molecule from the gastrointestinal tract. Curr Opin Pharmacol 2002; 2: 665–68.
Zhang JV, Ren PG, Aysian-Kretchmer O et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005; 310(5750): 996–9.
Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 2004; 286: 183–8.
Batter ham RL, Cowley MA, Small CJ et al. Gut hormone PYY (3–36) physiologically inhibits food intake. Nature 2002; 418: 650–4.
Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 2006; 7: 163–82.
Cigaina V, Saggioro A, Rigo V et al. Long-term effects of gastric pacing to reduce feed intake in swine. Obes Surg 1996; 6: 250–3.
Yin JY, Chen JD. Retrograde gastric electrical stimulation reduces food intake and weight in obese rats. Obes Res 2005; 13: 1580–7.
Shikora SA. Implantable gastric stimulation for the treatment of severe obesity. Obes Surg 2004; 14: 545–8.
Miller KA. Implantable electrical gastric stimulation to treat morbid obesity in the human: operative technique. Obes Surg 2002; 12 (Suppl 1): 17S–20S.
Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obese Surg 2002; 12 (Suppl 1): 12S–16S.
Sun Y, Chen JD. Intestinal electrical stimulation decreases fat absorption in rats: Therapeutic potential for obesity. Obes Res 2004; 12: 1235–42.
Liu S, Hou XH, Chen JD. Therapeutic Potential of duodenal electrical stimulation for obesity: Acute effects on gastric emptying and water intake. Am J Gastroenterol 2004; 99: 1–5.
Xu XH, Zhu HB, Chen JD. Pyloric electrical stimulation reduces food intake by inhibiting gastric motility in dogs. Gastroenterology 2005; 128: 43–50.
Ouyang H, Yin JY, Chen JD. Inhibitory effects of chronic gastric electrical stimulation on food intake and weight and their possible mechanisms. Dig Dis Sci 2003; 48: 698–705.
Xing JH, Chen JD. Effects and mechanisms of longpulse gastric electrical stimulation on canine gastric tone and accommodation. Neurogastroenterol Motil 2006; 18: 136–43.
Qin C, Sun Y, Chen JD et al. Gastric electrical stimulation modulates neuronal Activity in nucleus tractus solitarii in rats. Auton Neurosci 2005; 29: 119: 1–8.
Tang M, Zhang J, Chen JD. Central mechanisms of gastric electrical stimulation involving neurons in the paraventricular nucleus of the hypothalamus. Obes Surg 2006; 16: 344–52.
Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res 2003; 11: 1456–62.
De Luca M, Segato G, Busetto L et al. Progress in implantable gastric stimulation: summary of results of the European multi-center study. Obes Surg 2004; 14 (Suppl 1): S33–S39.
Xing JH, Lei Y, Ancha HR et al. Effect of acute electrical stimulation on the systemic release of hormones and plasma glucose in dogs. Dig Dis Sci (in press).
Tang M, Zhang J, Sun XR et al Implantable gastric stimulation alters expression of oxytocin- and orexincontaining neurons in the hypothalamus of rats. Obes Surg 2006; 16: 762–9.
Zhang J, Chen JD. Gastric electrical stimulation for obesity: Is there a need for a new generation device? Gastroenterology 2006; 130: A–248.
Hofbauer KH, Jensen BL, Kurtz A et al. Tissue hypoxygenation activates the Adrenomedullin system in vivo. Am J Physiol Regul Integr Comp Physiol 2000; 278: R513–519.
Lee HM, Wang G, Englander EW et al. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine and dietary manipulations. Endocrinology 2002; 143: 185–90.
Druce MR, Small CJ, Bloom SR. Gut peptides regulating satiety. Endocrinology 2004; 145: 2660–5.
Asakawa A, Inui A, Kaga T et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003; 52: 947–52.
Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Current Drug Targets 2005; 6: 153–69.
Bagnasco M, Tulipano G, Melis MR et al. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul Pept 2003; 111: 161–7.
Dornonville de la Cour C, Lindqvist A, Egecioglu E et al. Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut 2005; 54: 907–13.
Geloneze B, Tambascia MA, Pilla VF et al. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg 2003; 3: 17–22.
Smith GP, Gibbs J. The development and proof of the CCK hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD et al, eds. Multiple Cholecystokinin Receptors in the CNS. Oxford: Oxford Univ Press, 1992: 166–82.
Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973; 84: 488–95.
Ballinger A, McLaughlin L, Medbak S et al. Cholecystokinin is a satiety hormone in humans at physiological postprandial plasma concentrations. Clin Sci 1995; 89: 375–81.
Hanusch-Enserer U, Roden M. News in gut-brain communication: a role of peptide YY (PYY) in human obesity and following bariatric surgery. Eur J Clin Invest 2005; 35: 425–30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., McNearney, T.A. & Chen, J.D.Z. Gastric/Intestinal Electrical Stimulation Modulates Appetite Regulatory Peptide Hormones in the Stomach and Duodenum in Rats. OBES SURG 17, 406–413 (2007). https://doi.org/10.1007/s11695-007-9049-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9049-7