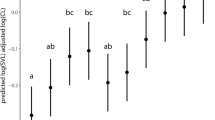
Abstract
Claws are the most common attachment mechanism in vertebrates. The comparative anatomy and morphology of claws has been studied mainly in reptiles and birds. However, as far as we know, studies focusing on turtles’ claws are lacking. Turtles occupy a wide range of habitats, from aquatic to terrestrial, and vary in form and behavior, being an ideal model organism for ecomorphological studies. We performed qualitative and quantitative analyses to find a relationship between morphological variation and both ecological factors and phylogenetic constraints that could have driven the evolution of turtles’ claws. The claws of 35 adult turtle and tortoise specimens of 12 species of testudines with different locomotor modes were compared. Our data show several convergence traits in claw shape, with convergence being reinforced by the low phylogenetic signal exhibited by most characters. We propose that claw morphology in turtles is mainly associated with some mechanical functions, such as freshwater-swimming, bottom-walking and tearing of prey.




Similar content being viewed by others
Change history
19 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11692-022-09586-w
References
Andreu, A. C., Díaz-Paniagua, C., Keller, C., Slimani, T., & El Mouden, H. (2004). Testudo [graeca] graeca. Manouria, 7(22), 17–18.
Angielczyk, K. D., Burroughs, R. W., & Feldman, C. R. (2015). Do turtles follow the rules? Latitudinal gradients in species richness, body size, and geographic range area of the world’s turtles. Journal of Experimental Zoology, 324B, 270–294.
Arnold, S. J. (1983). Morphology, performance and fitness. American Zoologist, 23, 347–361.
Beggs, K., Young, J., Georges, A., & West, P. (2011). Ageing the eggs and embryos of the pig-nosed turtle, Carettochelys nsculpta (Chelonia: Carettochelydidae), from northern Australia. Canadian Journal of Zoology, 78(3), 373–392.
Benson, R. B. J., Domokos, G., Várkonyi, P. L., & Reisz, R. R. (2011). Shell geometry and habitat determination in extinct and extant turtles (Reptilia: Testudinata). Paleobiology, 37, 547–562.
Bent, A. C. (1919). Life histories of North American diving birds. Dover.
Biewener, A. A. (2003). Animal Locomotion. Oxford University Press.
Björklund, M., & Merilä, J. (1993). Morphological differentiation in Carduelis finches: Adaptive vs. constraint models. Journal of Evolutionary Biology, 6, 359–373.
Blob, R. W., Mayerl, C. J., Rivera, A. R. V., Rivera, G., & Young, V. K. H. (2016). On the fence” versus “all in”: Insights from turtles for the evolution of aquatic locomotor specializations and habitat transitions in tetrapod vertebrates. Integrative and Comparative Biology, 56(6), 1310–1322.
Burnham, K. K., Burnham, J. L., Johnson, J. A., & Huffman, A. (2021). Migratory movements of Atlantic puffins Fratercula arctica naumanni from high Arctic Greenland. PLoS ONE, 16(5), e0252055. https://doi.org/10.1371/journal.pone.0252055
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference a practical information–theoretic approach (2nd ed.). Springer-Verlag.
Butler, M. A., & King, A. A. (2004). Phylogenetic comparative analysis: A modeling approach for adaptive evolution. The American Naturalist, 164, 6.
Butterfield, T., Olson, M., Beck, D., & Macip-Ríos, R. (2020). Morphology, performance, and ecology of three sympatric turtles in a tropical dry forest. Copeia, 108(4), 957–966. https://doi.org/10.1643/CE-18-165
Cassano, M. J. (2017). Restricción de la distribución de Mesoclemmys vanderhaegei. Cuadernos De Herpetología, 31(1), 25–27.
Chebez, J. C., Rey, N. R., & Williams, J.D. (2005) Reptiles de los Parques Nacionales de la Argentina. Monografía, Literature of Latin America, (L.O.L.A.) Buenos Aires, Argentina, 76 pp.
Claude, J., Paradis, E., Tong, H., & Auffray, J.-C. (2003). Ageometric morphometric assessment of the effects of environ-ment and cladogenesis on the evolution of the turtle shell. Biological Journal of the Linnean Society, 79, 485–501.
Claude, J., Pritchard, P. C. H., Tong, H., Paradis, E., & Auffray, J.-C. (2004). Ecological Correlates and evolutionary divergence in the skull of turtles: A geometric morphometric assessment. Systematic Biology, 53(6), 933–948.
D’Amore, D. C., Clulow, S., Doody, J. S., Rhind, D., & McHenry, C. R. (2018). Claw morphometrics in monitor lizards: Variable substrate and habitat use correlate to shape diversity within a predator guild. Ecology and Evolution, 8, 6766–6778.
Evers, S. W., & Benson, R. B. (2018). A new phylogenetic hypothesis of turtles with implications for the timing and number of evolutionary transitions to marine lifestyles in the group. Palaeontology, 62(5), 1–42.
Fabre, A.-C., Cornette, R., Goswami, A., & Peigné, S. (2015). Do constraints associated with the locomotor habitat drive the evolution of forelimb shape? A case study in mustelid carnivorans. Journal of Anatomy, 226, 596–620.
Feduccia, A. (1993). Evidence from claw geometry indicating arboreal habits of Archaeopteryx. Science, 259, 790–793.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1–15.
Ferreira, G. S., Bronzati, M., Langer, M. C., & Sterli, J. (2018). Phylogeny, biogeography and diversifcation patterns of side-necked turtles (Testudines: Pleurodira). Royal Society Open Science, 5, 171–773.
Gaffney, E. S. (1990). The comparative osteology of the Triassic turtle Proganochelys. Bulletin of the American Museum of Natural History, 194, 1–263.
Guillon, J.-M., Guéry, L., Hulin, V., & Girondot, M. (2012). A large phylogeny of turtles (Testudines) using molecular data. Contributions to Zoology, 81(3), 147–158.
Hahn, S., Dimitrov, D., Rehse, S., Yohannes, E., & Jenni, L. (2014). Avian claw morphometry and growth determine the temporal pattern of archived stable isotopes. Journal of Avian Biology, 45, 202–207.
Harmon, L. J., Losos, J. B., Jonathan Davies, J., Gillespie, R. G., Gittleman, J. L., Bryan, J. W., Kozak, K. H., McPeek, M. A., Moreno-Roark, F., Near, T. J., Purvis, A., Ricklefs, R. E., Schluter, D., Schulte, J. A., Jr., Seehausen, O., Sidlauskas, B. L., Torres-Carvajal, O., Weir, J. T., & Mooers, A. Ø. (2010). Early bursts of body size and shape evolution are rare in comparative data. Evolution, 64, 2385–2396.
Herrel, A., McBrayer, L. D., & Larson, P. M. (2007). Functional basis for sexual differences in bite force in the lizard Anolis carolinensis. Biological Journal of the Linnean Society, 91, 111–119.
Herrel, A., Meyers, J., & Vanhooydonck, B. (2001). Correlations between habitat use and body shape in a phrynosomatid lizard (Urosaurus ornatus): A population-level analysis. Biological Journal of the Linnean Society, 74, 305–314.
Hildebrand, M. (1995). Analysis of vertebrate structure. John Wiley & Sons Inc.
Hornung, M. N. (1982). Burrows and burrowing of the puffin (Fratercula arctica). Bangor: Institute of Terrestrial Ecology.
Jaffe, A. L., Slater, G. J., & Alfaro, M. E. (2011). The evolution of island gigantism and body size variation in tortoises and turtles. Biology Letters, 7, 558–561.
James, F. C. (1982). The ecological morphology of birds: A review. Annales Zoologici Fennici, 19, 265–275.
Kiester, A. R., & Willey, L. L. (2015). Terrapene carolina (linnaeus 1758) –eastern box turtle, common box turtle conservation biology of freshwater turtles and tortoises. Chelonian Research Monographs., 5, 085.
Klingenberg, C. P., & Ekau, W. (1996). A combined morphometric and phylogenetic analysis of an ecomorphological trend: Pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biological Journal of the Linnean Society, 59, 143–177.
Lande, R., & Arnold, S. J. (1983). The measurement of selection on correlated characters. Evolution, 36, 1210–1226.
Li, C., Wu, X. C., Rieppel, O., Wang, L.-T., & Zhao, L.-J. (2008). An ancestral turtle from the Late Triassic of southwestern China. Nature, 456, 497–501.
López, M. S., Sione, W. F., Leynaud, G. C., Prieto, Y., & Manzano, A. S. (2013). How far away from water? Terrestrial dispersal and nesting sites of the freshwater turtle Phrynops hilarii in the floodplain of the Paraná River (Argentina). Zoological Science, 30, 1063–1069.
Losos, J. B. (1990). Ecomorphology, performance capability and scaling of West Indian Anolis lizards: An evolutionary analysis. Ecological Monographs, 60, 369–388.
Losos, J. B. (2009). Lizards in an evolutionary tree: Ecology and adaptive radiations of anoles. Univ. of California Press.
Mann, A., Dudgeon, T. W., Henrici, A. C., Berman, D. S., & Pierce, S. E. (2021). Digit and ungual morphology suggest adaptations for scansoriality in the late carboniferous eureptile Anthracodromeus longipes. Frontiers in Earth Science, 9, 675337. https://doi.org/10.3389/feart.2021.67533
Mao, S., Zhang, M., Liu, J., et al. (2015). Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Scientific Reports, 5, 16116.
Martin, P., & Bateson, P. (1993). Measuring behavior: An introductory guide (2nd ed.). Cambridge University Press.
Norris, D., Pitman, N., Gonzalez, J. M., Torres, E., Pinto, F., Collado, H., Concha, W., Thupa, R., Quispe, E., Pérez, J., & Flores del Castillo, J. C. (2011). Abiotic modulators of Podocnemis unifilis (Testudines: Podocnemididae) abundances in the Peruvian Amazon. Zoologia (curitiba), 28(3), 343–350.
Orme, C. D. L., Freckleton, R. P., Thomas, G. H., Petzoldt, T., Fritz, S. A., & Isaac, N. B. J. (2012). Carper: Comparative analyses of phylogenetics and evolution in R. Methods Ecology and Evolution, 3, 145–151.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioninformatics, 20, 289–290.
Prado, W. S., Waller, T., Albareda, D. A., Cabrera, M. R., Etchepare, E. G., Giraudo, A., González Carman, V., Prosdocimi, L., & Richard, E. (2012). Categorización del estado de conservación de las tortugas de la República Argentina. Cuadernos De Herpetología, 26(1), 375–388.
R Development Core Team. (2022). R: a language and environment for statistical computing. Vienna: R foundation for statistical computing.
Revell, L. J. (2009). Size-correction and principal components for interspecific comparative studies. Evolution, 63, 3258–3268.
Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Richard, E., Waller, T., Aprile, G., Bertonatti, C., Carcacha, H., Fallabino, A. Frazier, J. G., Giraudo, A., & Tracchia, A. (2000). Categorización de las Tortugas de Argentina Categorización de las Tortugas de Argentina. In: E. O. Lavilla, E. Richardand, & G. J Scrocchi (eds). Categorización de los Anfibios y Reptiles de la República Argentina. Tucumán: Asociación Herpetológica Argentina.
Rivera, G. (2008). Ecomorphological variation in shell shape of thefreshwater turtle Pseudemys concinna inhabiting different aquatic flow regimes. Integrative aNd Comparative Biology, 48, 769–787.
Rivera, G., Davis, J. N., Godwin, J. C., & Adams, D. C. (2014). Repeatability of habitat-associated divergence in shell shape of turtles. Evolutionary Biology, 41, 29–37.
Schliep, K. P. (2011). phangorn: Phylogenetic analysis in R. Bioinformatics, 27, 592–593.
Schoch, R. R., & Sues, H.-D. (2018). Osteology of the middle triassic stem-turtle Pappochelys rosinae and the early evolution of the turtle skeleton. Journal of Systematic Palaeontology, 16(11), 927–965.
Schoch, R. R., & Sues, H.-D. (2019). The origin of the turtle body plan: Evidence from fossils and embryos. Paleontology, 63(3), 375–393.
Schultz, T., & Doody, S. (2004). Varanus mitchelli. In E. R. Pianka, D. R. King, & R. A. King (Eds.), Varanoid Lizards of the World (pp. 416–422). Indiana University Press.
Shine, R. (1986). Food habits, habitats and reproductive biology of four sympatric species of varanid lizards in tropical Australia. Herpetologica, 42, 346–360.
Stanner, M. (2010). Mammal-like feeding behavior of Varanus salvator and its conservational implications. Biawak, 4(4), 128–131.
Stein, B. R. (2000). Morphology of Subterranean Rodents. In E. A. Lacey, J. L. Patton, & G. N. Cameron (Eds.), Life underground: The biology of subterranean rodents (pp. 19–61). University of Chicago Press.
Thomson, T. J., & Motani, R. (2021). Functional morphology of vertebrate claws investigated using functionally based categories and multiple morphological metrics. Journal of Morphology, 282, 449–471. https://doi.org/10.1002/jmor.21317
Tulli, M. J., Abdala, V., & Cruz, F. B. (2011). Relationships among morphology, clinging performance and habitat use in Liolaemini lizards. Journal of Evolutionary Biology, 24, 843–855.
Tulli, M. J., Carrizo, L. V., & Samuels, J. X. (2016). Morphological variation of the forelimb and claw in Neotropical Sigmodontine rodents (Rodentia: Cricetidae). Journal of Mammalian Evolution, 23, 81–91.
Tulli, M. J., Cruz, F. B., Herrel, A., Vanhooydonck, B., & Abdala, V. (2009). The interplay between claw morphology and microhabitat use in neotropical iguanian lizards. Zoology, 112, 379–392.
Watanabe, A., Fabre, A. C., Felice, R. N., Maisano, J. A., Müller, J., Herrel, A., & Goswami, A. (2019). Ecomorphological diversification in squamates from conserved pattern of cranial integration. Proceedings of the National Academy of Sciences of the United States of America, 116, 14688–14697.
Willey, J. S., & Blob, R. W. (2004). Tail kinematics of juvenile common snapping turtles during aquatic walking. Journal of Herpetology, 38(3), 360–369.
Williams, E. E. (1972). The origin of faunas. Evolution of lizard congeners in a complex island fauna: A trial analysis. Evolutionary Biology, 6, 47–89.
Wyneken, J. (2001). The Anatomy of Sea Turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470.
Wyneken, J., & Salmon, M. (2020). Linking ecology, morphology, and behavior to conservation: Lessons learned from studies of sea turtles. Integrative and Comparative Biology, 60(2), 440–455.
Zani, P. (2000). The comparative evolution of lizard claw and toe morphology and clinging performance. Journal of Evolutionary Biology, 13, 316–325.
Acknowledgements
We are very thankful to Marta Cánepa and Sonia Kretzschmar from Herpetological Collection of the Fundación Miguel Lillo for allowing access to herpetological collections, Herpetologial collection CICyTTP-Conicet, Paola Soñez and Miriam Vera from Laboratorio de Genética Evolutiva, Misiones, for making the study of some additional specimens possible. We acknowledge Belén Haad and Juan Carlos Stazonelli for her help. The study was supported by PICT 2016-2772, PICT 2018 382; PIP 0389 to VA.
Funding
PICT 2016–2772, PICT 2018 382; PIP 0389 to VA.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: MJT. AM. and VA. Collected data: MJT and AM., and analyzed data MJT. Drafting the article: all authors. Critical revision of the article: all authors. Final approval of the version to be published: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Data Availability
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
Consent to Participate
All authors are agreeing.
Consent for Publication
All authors are agreeing.
Additional information
The original online version of this article was revised: “In this article the affiliation details for author V. Abdala updated as Instituto de Biodiversidad Neotropical, UNT-CONICET, Cátedra de Biología General, Facultad de Ciencias Naturales E IML-Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina and in the sentence beginning Claw morphology of turtles appears in this article, the term Most claw traits showed a relationship with a phylogeny (λ close to 0 and not significant). Instead of 0 is 1 and one claw traits (CHDII) showed a λ close to 1 and significant, instead of 1 is 0”
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tulli, M.J., Manzano, A. & Abdala, V. Is the Shape of Turtle Claws Driven by Locomotor Modes?. Evol Biol 49, 424–432 (2022). https://doi.org/10.1007/s11692-022-09580-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-022-09580-2