Abstract
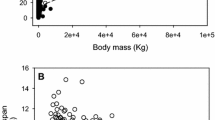
We carried out 42 lifetime studies of male and female crickets (Acheta domesticus) treated with different diets, nutritional supplements and dietary restriction (84 groups). Maximal longevity of controls averaged ~118 days and various treatments obtained lifespans of 86–257 days. Overall, greater longevity was most strongly associated with slower growth and delayed maturation. Early nymphal mortality and early fasting stress were also associated with life extension. The opposite associations of growth rate and stress resistance with maximal life span are consistent with modulation of aging by the target of rapamycin (growth) and forkhead transcription factors (stress resistance). Unlike inter-specific comparisons or intra-specific comparisons of species differentiated into strains or breeds, body size did not emerge as a strong predictor of cricket longevity. Rather, targeted body size was strongly compensated for in crickets whereas most genetically differentiated breeds of mice, rats, horses and dogs express breed-specific sizes and growth rates. Both extension of the juvenile period and adult lifetime contributed to increases in maximal longevity of crickets, although for extreme life extension, extended immaturity was a greater factor than adult life span. We discuss the inter- and intra-specific allometry of life histories from a perspective of aging and suggest that inter-specific variance may particularly reflect exogenous mortality risks whereas intra-specific aging is dominated by constraints on productivity.









Similar content being viewed by others
References
Aksenov, V., Long, J., Liu, J., Szechtman, H., Khanna, P., & Matravadia, S. (2011). A complex dietary supplement augments spatial learning, brain mass, and mitochondrial electron transport chain activity in aging mice. Age. doi:10.1007/s11357-011-9325-2 (Ahead of print).
Aksenov, V., Long, J., Lokuge, S., Foster, J. A., Liu, J., & Rollo, C. D. (2010). A dietary supplement ameliorates locomotor, neurotransmitter and mitochondrial aging. Experimental Biology and Medicine, 335, 66–76.
Archer, C. R., Royle, N., South, S., Selman, C., & Hunt, J. (2009). Nutritional geometry provides food for thought. Journals of Gerontology A Biological Science and Medical Science, 64A, 956–959.
Austad, S. N. (2010). Animal size, metabolic rate, and survival, among and within species. In N. S. Wolf (Ed.), The comparative biology of aging (Vol. 27, pp. 27–41). New York: Springer.
Bateman, P. W., Ferguson, J. W. H., & Yetman, C. A. (2005). Courtship and copulation, but not ejaculates, reduce the longevity of female field crickets (Gryllus bimaculatus). Journal of Zoology,. doi:10.1111/j.1469-7998.2006.00054.x.
Behmer, S. T. (2009). Insect herbivore nutrient regulation. Annual Review of Entomology, 54, 165–187.
Booth, D. T., & Kiddell, K. (2007). Temperature and the energetics of development in the house cricket (Acheta domesticus). Journal of Insect Physiology, 53, 950–953.
Bowen, R. L., & Atwood, C. S. (2004). Living and dying for sex. Gerontology, 50, 265–290.
Burpee, D. M., & Sakaluk, S. K. (1993). Repeated matings offset costs of reproduction in female crickets. Evolutionary Ecology, 7, 240–250.
Calder, W. A. (1984). Size, function, and life history. Cambridge: Harvard University Press.
Carey, J. R., Harshman, L., Liedo, P., Muller, H. G., Wang, J. L., & Zhang, Z. (2008). Longevity-fertility trade-offs in the Tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell, 7, 470–477.
Case, T. J. (1978). On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Quarterly Review of Biology, 53, 243–276.
Castrillo, J. I., Zeef, L. A., Hoyle, D. C., Zhang, N., Hayes, A., Gardner, D. C. J., et al. (2007). Growth control of the eukaryote cell: A systems biology study in yeast. Journal of Biology, 6, 4. doi:10.1186/jbiol54.
Charnov, E. L. (1993). Life history invariants. Oxford: Oxford University Press.
Cutler, R. G. (1984a). Antioxidants, aging and longevity. In W. A. Pryor (Ed.), Free radicals in biology (Vol. VI, pp. 371–428). New York: Academic Press.
Cutler, R. G. (1984b). Evolutionary biology of aging and longevity in mammalian species. In J. E. Johnson (Ed.), Aging and cell function (pp. 1–147). New York: Plenum Press.
Dabour, N., Bando, T., Nakamura, T., Miyawaki, K., Mito, T., Ohuchi, H., et al. (2011). Cricket body size is altered by systemic RNAi against insulin signaling components and epidermal growth factor receptor. Development Growth and Differentiation, 53, 857–869.
de Magalhaes, J. P., & Church, G. M. (2005). Genomes optimize reproduction: Aging as a consequence of the developmental program. Physiology, 20, 252–259.
de Magalhaes, J. P., Costa, J., & Church, G. M. (2007). An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. Journals of Gerontology, 62A, 149–160.
Dobson, F. S. (2007). A life-style view of life history variation. Proceedings of the National Academy of the United States of America, 104, 17565–17566.
Esperk, T., Tammaru, T., & Nylin, S. (2007). Intraspecific variability in number of larval instars in insects. Journal of Economic Entomology, 100, 627–645.
Fanson, B. G., Weldon, C. W., Perez-Staples, D., Simpson, S. J., & Taylor, P. W. (2009). Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell, 8, 514–523.
Finch, C. E. (1976). The regulation of physiological changes during mammalian aging. Quarterly Review of Biology, 51, 49–83.
Galis, F., Sluijs, I. V. D., Dooren, T. J. M. V., Metz, J. A. J., & Nussbaumer, M. (2007). Do large dogs die young? Journal of Experimental Zoology, 308B, 119–126.
Glazier, D. S. (1999). Trade-offs between reproductive and somatic (storage) investments in animals: A comparative test of the Van Noodwijk and De Jong model. Evolutionary Ecology, 13, 539–555.
Glazier, D. S. (2010). A unifying explanation for diverse metabolic scaling in animals and plants. Biological Reviews, 85, 111–138.
Grandison, R. C., Piper, M. D. W., & Partridge, L. (2009). Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature, 462, 1061–1064.
Hamilton, M. J., Davidson, A. D., Sibly, R. M., & Brown, J. H. (2011). Universal scaling of production rates across mammalian lineages. Proceedings of the Royal Society B, 278, 560–566.
Hunt, J., Brooks, R., Jennions, M. D., Smith, M. J., Bentsen, C. L., & Bussiere, L. F. (2004). High-quality male field crickets invest heavily in sexual display but die young. Nature, 432, 1024–1027.
Hunt, J., Jennions, M. D., Spyrou, N., & Brooks, R. (2006). Artificial selection on male longevity influences age-dependent reproductive effort in the black field cricket Teleogryllus commodus. American Naturalist, 168, E72–E86.
Ja, W. W., Carvalho, G. B., Zid, B. M., Mak, E. M., Brummel, T., & Benzer, S. (2009). Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proceedings National Academy of Sciences of the United States of America, 106, 18633–18637.
Kearney, M., Simpson, S. J., Raubenheimer, D., & Helmuth, B. (2010). Modelling the ecological niche from functional traits. Proceedings of the Royal Society of London B Biological Sciences, 365, 3469–3483.
Kirkwood, J. K. (1985). The influence of size on the biology of the dog. Journal of Small Animal Practice, 26, 97–110.
Kirkwood, T. B. L. (2008). A systematic look at an old problem. Nature, 451, 644–647.
Kirkwood, T. B. L., & Holliday, R. (1979). The evolution of ageing and longevity. Proceedings of the Royal Society of London B, 205, 531–546.
Lee, K. P., Simpson, S. J., Clissold, F. J., Brooks, R., Ballard, J. W. O., Taylor, P. W., et al. (2008). Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proceedings of the National Academy of the United States of America, 105, 2498–2503.
Lemon, J. A., Boreham, D. R., & Rollo, C. D. (2003). A dietary supplement abolishes age-related cognitive decline in transgenic mice expressing elevated free radical processes. Experimental Biology and Medicine, 228, 800–810.
Lemon, J. A., Boreham, D. R., & Rollo, C. D. (2005). A complex dietary supplement extends longevity of mice. Journals of Gerontology, 60A, 275–279.
Lemon, J. A., Rollo, C. D., & Boreham, D. R. (2008a). Elevated DNA damage in a mouse model of oxidative stress: Impacts of ionizing radiation and a protective dietary supplement. Mutagenesis, 23, 473–482.
Lemon, J. A., Rollo, C. D., McFarlane, N. M., & Boreham, D. R. (2008b). Radiation-induced apoptosis in mouse lymphocytes is modified by a complex dietary supplement: The effect of genotype and gender. Mutagenesis, 23, 465–472.
Lyn, J., Naik, W., Aksenov, V., & Rollo, C. D. (2011). Development of the cricket Acheta domesticus as a model of aging. AGE, 33, 509–522.
Maklakov, A. A., Hall, M. D., Simpson, S. J., Dessmann, J., Clissold, F. J., Zajitschek, F., et al. (2009). Sex differences in nutrient-dependent reproductive ageing. Aging Cell, 8, 324–330.
Maklakov, A. A., Simpson, S. J., Zajitschek, F., Hall, M. D., Dessmann, J., Clissold, F., et al. (2008). Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology, 18, 1062–1066.
McCay, C. M., Pope, F., & Lunsford, W. (1956). Experimental prolongation of the life span. Bulletin of the New York Academy of Medicine, 32, 91–101.
McFarlane, J. E., Amit, I., & Sixeves, E. (1984). Studies on the group effect in Acheta domesticus (L.) using artificial diets. Journal of Insect Physiology, 30, 103–107.
Miller, R. A., Harper, J. M., Dysko, R. C., Durkee, S. J., & Austad, S. N. (2002). Longer life spans and delayed maturation in wild-derived mice. Experimental Biology and Medicine, 227, 500–508.
Okada, K., Pitchers, W. R., Sharma, M. D., Hunt, J., & Hosken, D. J. (2010). Longevity, calling effort, and metabolic rate in two populations of cricket. Behavioral Ecology and Sociobiology, 65, 1773–1778.
Ooka, H., Segall, P. E., & Timiras, P. S. (1988). Histology and survival in age-delayed low-tryptophan-fed rats. Mechanisms of Ageing and Development, 43, 79–98.
Ophir, A. G., Schrader, S. B., & Gilooly, J. F. (2010). Energetic cost of calling: General constraints and species-specific differences. Journal of Evolutionary Ecology, 23, 1564–1569.
Partridge, L., Piper, M. D. W., & Mair, W. (2005). Dietary restriction in Drosophila. Mechanisms of Ageing and Development, 126, 938–950.
Patronek, G. J., Waters, D. J., & Glickman, L. T. (1997). Comparative longevity of pet dogs and humans: Implications for gerontology research. Journals of Gerontolology A Biological Sciences and Medical Sciences, 52A, B171–B178.
Pearl, R. (1928). The rate of living. London: University of London Press.
Peters, R. H. (1983). The ecological implications of body size. Cambridge: Cambridge University Press.
Pletcher, S. D., Khazaeli, A. A., & Curtsinger, J. W. (2000). Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. Journals of Gerontology, 55A, B381–B389.
Price, P. W. (1974). Strategies for egg production. Evolution, 28, 76–84.
Renucci, M., Strambi, C., Strambi, A., Augier, R., & Charpin, P. (1990). Ovaries and regulation of juvenile hormone titer in Acheta domesticus L. (Orthoptera). General and Comparative Endocrinology, 78, 137–149.
Ricklefs, R. E. (2000). Intrinsic aging-related mortality in birds. Journal of Avian Biology, 31, 103–111.
Ricklefs, R. E. (2010). Life-history connections to rates of aging in terrestrial vertebrates. Proceedings of the National Academy of the United States of America, 107, 10314–10319.
Rollo, C. D. (1994). Phenotypes: Their epigenetics, ecology and evolution. London: Chapman and Hall.
Rollo, C. D. (2002). Growth negatively impacts the life span of mammals. Evolution and Development, 4, 55–61.
Rollo, C. D. (2007a). The evolutionary ecology of body size with special reference to allometry and survivorship. In T. Samaras (Ed.), Human body size and the laws of scaling (pp. 213–234). New York: Nova Scientific Publishing.
Rollo, C. D. (2007b). Multidisciplinary aspects of regulatory systems relevant to multiple stressors: Aging, xenobiotics and radiation. In C. Mothersill (Ed.), Multiple stressors: A challenge for the future (pp. 185–224). New York: Springer.
Rollo, C. D. (2010). Aging and the mammalian regulatory triumvirate. Aging and Disease, 1, 105–138. http://www.aginganddisease.org/issue-2.html.
Rollo, C. D. (2011). Circadian redox regulation. In K. Pantopoulos & H. M. Schipper (Eds.), Principles of free radical biomedicine. New York: Nova Scientific Publishing.
Samaras, T. (Ed.). (2007). Human body size and the laws of scaling. New York: Nova Scientific Publishing.
Schmaulhausen, I. I. (1949). Factors of evolution: The theory of stabilizing selection. Philadelphia: Blakiston.
Schmidt-Nielson, K. (1984). Scaling: Why is animal size so important?. Cambridge: Cambridge University Press.
Sibly, J. R., & Brown, J. H. (2007). Effects of body size and lifestyle on evolution of mammal life histories. Proceedings of the National Academy of Sciences, 104, 17707–17712.
Simpson, S. J., & Raubenheimer, D. (2010). The nutritional geometry of aging. In A. V. Everitt, S. I. S. Rattan, D. G. Le Couteur, & R. de Cabo (Eds.), Calorie restriction, aging and longevity (pp. 111–122). Dordrecht: Springer.
Skorupa, D. A., Dervisefendic, A., Zwiener, J., & Pletcher, S. D. (2008). Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell, 7, 478–490.
Sutter, N. B., Bustamante, C. D., Chase, K., Gray, M. M., Zhao, K., Zhu, L., et al. (2007). A single IGF1 allele is a major determinant of small size in dogs. Science, 316, 112–115.
Tanaka, S. (2001). Effects of suppressed oviposition activity and flight muscle histolysis on food consumption and ovarian development in a wing-dimorphic cricket: An explanation for sporadic conclusions related to physiological trade-offs. Journal of Insect Physiology, 47, 83–94.
Tu, M. P., & Tatar, M. (2003). Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell, 2, 327–333.
Van Noordwijk, A. J., & de Jong, G. (1986). Acquisition and allocation of resources: Their influence on variation in life history tactics. American Naturalist, 128, 137–142.
Wagner, W. E., Jr, & Harper, C. J. (2003). Female life span and fertility are increased by the ejaculates of preferred males. Evolution, 57, 2054–2066.
Wagner, W. E., Jr, Kelley, R. J., Tucker, K. R., & Harper, C. J. (2001). Females receive a life-span benefit from male ejaculates in a field cricket. Evolution, 55, 994–1001.
Wedell, N., Tregenza, T., & Simmons, L. W. (2008). Nuptial gifts fail to resolve a sexual conflict in an insect. BMC Evolutionary Biology, 8, 204. doi:10.1186/1471-2148-8-204.
Williams, G. C. (1957). Pleiotropy, natural selection and the evolution of senescence. Evolution, 11, 398–411.
Woodring, J. P. (1983). Control of moulting in the house cricket, Acheta domesticus. Journal Insect Physiology, 29, 461–464.
Woodring, J. P., Roe, R. M., & Clifford, C. W. (1977). Relation of feeding, growth, and metabolism to age in the larval, female house cricket. Journal of Insect Physiology, 23, 207–212.
Yu, B. B. (1996). Aging and oxidative stress: Modulation by dietary restriction. Free Radical Biology and Medicine, 21, 651–668.
Zajitschek, F., Bonduriansky, R., Zajitschek, S. R. K., & Brooks, R. C. (2009a). Sexual dimorphism in life history: Age, survival, and reproduction in male and female field crickets Teleogryllus commodus under seminatural conditions. American Naturalist, 173, 792–802.
Zajitschek, F., Hunt, J., Jennions, M. D., Hall, M. D., & Brooks, R. C. (2009b). Effects of juvenile and adult diet on ageing and reproductive effort of male and female black field crickets, Teleogryllus commodus. Functional Ecology, 23, 602–611.
Zwaan, B., Bijlsma, R., & Hoekstra, R. F. (1995). Direct selection on life span in Drosophila melanogaster. Evolution, 49, 649–659.
Acknowledgments
We thank Warren Fingrut, Sherif Yani, Alaif Naima, Sonya Sidhu, and Wida Naikkhwah for contributions to our research program related to this analysis. We also thank two anonymous reviewers for their very constructive direction. This research was funded by a grant to CDR from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Appendix I: Composition of diets
Appendix I: Composition of diets
-
Guinea pig diet Living World ® Extrusion Guinea Pig Diet: Protein 16%, Fat 3.5%, Fibre 12.5%, Ash 6%, Calcium 0.8%, Phosphorus 0.6%, Copper sulphate 15 mg/kg, Vitamin A 6000 IU/kg, Vitamin C 300 mg/kg, Vitamin D3 520 IU/kg, Vitamin E 24 IU/kg
-
Cricket Gutload © Repashy Superfoods: Protein 20%; Fat 4.5%; Fiber 10%; Calcium 8%; Phosphorous 0.5%; Vitamin D3 20 IU/g; Vitamin A 200 IU/g; Beta carotene 5 mg/g; Choline 6 mg/g; Vitamin C 2.5 mg/g; Vitamin E 0.1 mg/g; B vitamins (B1, B2, B3, B5, B6, B7, B12) 0.83 mg/g; Vitamin K 0.03 mg/g).
-
Cat food Petcurean Go! Natural ® Cat Food: Protein 32%, Fat 20%, Fibre 3%, Moisture 9%, Ash 5.9%, Omega −6 5.8%, Omega 3 0.86%, Taurine 0.19%, Magnesium 0.09%, Vitamin E 200 IU/Kg
-
Kitten food Purina Kitten Chow: Protein (Min) 40%, Fat 12.5%, Fibre 4%, Moisture 12%, Calcium 1.2%, Phosphorus 1.1%, Taurine 0.125%
-
Whey protein diet Whey protein mixed with Cricket Gutload© to achieve an overall level of 40% protein.
Rights and permissions
About this article
Cite this article
Lyn, J., Aksenov, V., LeBlanc, Z. et al. Life History Features and Aging Rates: Insights from Intra-specific Patterns in the Cricket Acheta domesticus . Evol Biol 39, 371–387 (2012). https://doi.org/10.1007/s11692-012-9160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-012-9160-0