Abstract
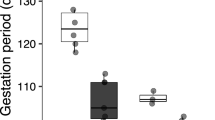
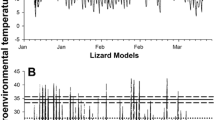
Pregnant female Zootoca vivipara select lower body temperatures than males or nonpregnant females, and this shift in the thermal preferendum is believed to be related to optimising the conditions for embryogenesis. Thus, subjecting embryos to the higher temperature selected by males and non-gravid females might have detrimental effects on embryonic development and on hatchling fitness, according to predictions of the “maternal manipulation” hypothesis on the evolution of viviparity. To test the role of gestation environment on embryonic development in oviparous Z. vivipara, we kept a number of gravid females at the temperature selected by non-gravid females in a laboratory thermal gradient, whereas control females were allowed to regulate their body temperature without restrictions. Developmental stage at oviposition was more advanced for embryos of the experimental clutches, which were heavier than those of the control group. Forced gestation temperature also affected hatching success (58.62% in the experimental treatment vs. 97.37% in the control group). In addition, hatchlings from females subjected to high temperatures during pregnancy were smaller, had shorter head length and performed worse in running trials. Our results fulfil the prediction of the “maternal manipulation” hypothesis, and suggest that the shift in female body temperature during pregnancy optimizes embryogenesis and hatchling phenotype by avoiding the negative effects of the high incubation temperatures preferred by non-gravid females.


Similar content being viewed by others
References
Andrews, R. M., Mathies, T., & Warner, D. A. (2000). Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetological Monographs, 14, 420–431.
Andrews, R. M., & Rose, B. R. (1994). Evolution of viviparity: constraints on egg retention. Physiological Zoology, 67, 1006–1024.
Angilletta, M. J., Jr, Winters, R. S., & Dunham, A. E. (2000). Thermal effects on the energetics of lizard embryos: implications for hatchling phenotypes. Ecology, 81, 2957–2968.
Arrayago, M. J., Bea, A., & Heulin, B. (1996). Hybridization experiment between oviparous and viviparous strains of Lacerta vivipara: A new insight into the evolution of viviparity in reptiles. Herpetologica, 52, 333–342.
Atkinson, D. (1994). Temperature and organism size: a biological law for ectotherms? Advances in Ecological Research, 25, 1–58.
Beuchat, C. A. (1988). Temperature effects during gestation in a viviparous lizard. Journal of Thermal Biology, 13, 135–142.
Birchard, G. F. (2004). Effects of incubation temperature. In D. C. Deeming (Ed.), Reptilian incubation: environment, evolution, and behavior (pp. 103–123). Nottingham: Nottigham University Press.
Blackburn, D. G. (1995). Saltationist and punctuated equilibrium models for the evolution of viviparity and placentation. Journal of Theoretical Biology, 174, 199–216.
Braña, F. (1993). Shifts in body temperature and escape behaviour of female Podarcis muralis during pregnancy. Oikos, 66, 216–222.
Braña, F. (1996). Sexual dimorphism in lacertid lizards: male head increase vs. female abdomen increase? Oikos, 75, 511–523.
Braña, F. (2008). Sex of incubation neighbours influences hatchling sexual phenotypes in an oviparous lizard. Oecologia, 156, 275–280.
Braña, F., Bea, A., & Arrayago, M. J. (1991). Egg retention in lacertid lizards: Relationships with reproductive ecology and the evolution of viviparity. Herpetologica, 47, 218–226.
Braña, F., & Ji, X. (2000). Influence of incubation temperature on morphology, locomotor performance, and early growth of hatchling wall lizards (Podarcis muralis). Journal of Experimental Zoology, 286, 422–433.
Braña, F., & Ji, X. (2007). The selective basis for increased egg retention: early incubation temperature determines hatchling phenotype in wall lizards (Podarcis muralis). Biological Journal of the Linnean Society, 92, 441–447.
Clobert, J., Oppliger, A., Sorci, G., Ernande, B., Swallow, J. G., & Garland, T., Jr. (2000). Trade-offs in phenotypic traits: endurance at birth, growth, survival, predation and susceptibility to parasitism in a lizard, Lacerta vivipara. Functional Ecology, 14, 675–684.
Daut, E. F., & Andrews, R. M. (1993). The effect of pregnancy on selected body temperatures of the viviparous lizard Chalcides ocellatus. Journal of Herpetology, 27, 6–13.
Dhouailly, D., & Saxod, R. (1974). Les stades du développement de Lacerta muralis Laurent entre la ponte et l’éclosion. Bulletin de La Societe Zoologique de France, 99, 489–494.
Du, W., & Ji, X. (2006). Effects of constant and fluctuating temperatures on egg survival and hatchling traits in the northern grass lizard (Takydromus septentrionalis, Lacertidae). Journal of Experimental Zoology, 305, 47–54.
Dufaure, J. P., & Hubert, J. (1961). Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Archives Anatomie Microscopie Morphologie Experemental, 50, 309–328.
Garland, T., Jr., Hankins, E., & Huey, R. B. (1990). Locomotor capacity and social dominance in male lizards. Functional Ecology, 4, 243–250.
Garland, T., Jr., & Losos, J. B. (1994). Ecological morphology of locomotor performance in squamate reptiles. In P. C. Wainwright & S. M. Reilly (Eds.), Ecological morphology. Integrative Organismal Biology (pp. 240–302). Chicago: University of Chicago Press.
Gvoždík, L., & Castilla, A. M. (2001). A comparative study of preferred body temperatures and critical thermal tolerance limits among populations of Zootoca viivpara (Squamata: Lacertidae) along an altitudinal gradient. Journal of Herpetology, 35, 486–492.
Gvoždík, L., & Van Damme, R. (2003). Evolutionary maintenance of sexual dimorphism in head size in the lizard Zootoca vivipara: A test of two hypotheses. Journal of Zoology, 259, 7–13.
Harlow, P. S. (1996). A harmless technique for sexing hatchling lizards. Herpetological Review, 27, 71–72.
Herrel, A., Van Damme, R., Vanhooydonck, B., & De Vree, F. (2001). The implications of bite performance for diet in two species of lacertid lizards. Canadian Journal of Zoology, 79, 662–670.
Heulin, B., Arrayago, M. J., Bea, A., & Braña, F. (1992). Caractéristiques de la coquille des œufs chez la souche hybride (ovipare x vivipare) du lézard Lacerta vivipara. Canadian Journal of Zoology, 70, 2242–2246.
Hubert, J. (1985). Embryology of the Squamata. In C. Gans & F. Billet (Eds.), Biology of the Reptilia, Development B (Vol. 15, pp. 1–34). New York: Wiley.
Ji, X., Lin, L., Luo, L., Lu, H., Gao, J., & Han, J. (2006). Gestation temperature affects sexual phenotype, morphology, locomotor performance, and growth of neonatal brown forest skinks, Sphenomorphus indicus.
Le Galliard, J. F., Clobert, J., & Ferrière, R. (2004). Physical performance and Darwinian fitness in lizards. Nature, 432, 502–505.
Mathies, T., & Andrews, R. (1995). Thermal and reproductive biology of high and low elevation populations of the lizard Sceloporus scalaris: implications for the evolution of viviparity. Oecologia, 104, 101–111.
Mathies, T., & Andrews, R. (1997). Influence of pregnancy on the thermal biology of the lizard, Sceloporus jarrovi: Why do pregnant females exhibit low body temperatures? Functional Ecology, 11, 498–507.
Packard, G. C. (1991). Physiological and ecological importance of water to embryos of oviparous reptiles. In D. C. Deeming & M. W. J. Ferguson (Eds.), Egg incubation: Its effects on embryonic development in birds and reptiles (pp. 213–228). Cambridge: Cambridge University Press.
Packard, G. C., & Packard, M. J. (1988). The physiological ecology of reptilian eggs and embryos. In C. Gans & R. B. Huey (Eds.), Biology of the reptilia (Vol. 16B, pp. 523–606). New York: Alan R Liss.
Panigel, M. (1956). Contribution a l’étude de l’ovoviviparité chez les reptiles: Gestation et parturition chez le lézard vivipare Zootoca vivipara. Annales des Sciences Naturelles, Zoologique, 18, 569–668.
Qualls, C. P., & Andrews, R. M. (1999). Maternal body volume constrains water uptake by lizard eggs in utero. Functional Ecology, 13, 845–851.
Robson, M. A., & Miles, D. B. (2000). Locomotor performance and dominance in male tree lizards, Urosaurus ornatus. Functional Ecology, 14, 38–344.
Rock, J., Andrews, R., & Cree, A. (2000). Effects of reproductive condition, season, and site on selected temperatures of a viviparous gecko. Physiological and Biochemical Zoology, 73, 344–355.
Rock, J., & Cree, A. (2003). Intraspecific variation in the effect of temperature on pregnancy in the viviparous gecko Hoplodactylus maculatus. Herpetologica, 59, 8–22.
Rodríguez-Díaz, T., & Braña, F. (2011). Plasticity and limitations of extended egg retention in oviparous Zootoca vivipara (Reptilia: Lacertidae). Biological Journal of the Linnean Society, 102, 75–82.
Rodríguez-Díaz, T., González, F., Ji, X., & Braña, F. (2010). Effects of incubation temperature on hatchling phenotypes in an oviparous lizard with prolonged egg retention: Are the two main hypotheses on the evolution of viviparity compatible? Zoology, 113, 33–38.
Shine, R. (1983). Reptilian reproductive modes: The oviparity-viviparity continuum. Herpetologica, 39, 1–8.
Shine, R. (1995). A new hypothesis for the evolution of viviparity in reptiles. The American Naturalist, 145, 809–823.
Shine, R. (2006). Is increased maternal basking an adaptation or a pre-adaptation to viviparity in lizards? Journal of Experimental Zoology, 305A, 524–535.
Shine, R., & Downes, S. J. (1999). Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia, 119, 1–8.
Shine, R., & Harlow, P. (1993). Maternal thermoregulation influences offspring viability in a viviparous lizard. Oecologia, 96, 122–127.
Shine, R., & Harlow, P. (1996). Maternal manipulation of offspring phenotypes via nest-site selection in an oviparous lizard. Ecology, 77, 1808–1817.
Sorci, G., & Clobert, J. (1997). Environmental effects on locomotor performance in the common lizard (Lacerta vivipara). Evolutionary Ecology, 11, 531–541.
Swain, R., & Jones, S. M. (2000). Maternal effects associated with gestation conditions in a viviparous lizard, Niveoscincus metallicus. Herpetological Monographs, 14, 432–440.
Van Damme, R., Bauwens, D., & Verheyen, R. F. (1991). The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Functional Ecology, 5, 507–517.
Verwaijen, R., Van Damme, R., & Herrel, A. (2002). Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Functional Ecology, 16, 842–850.
Wapstra, E. (2000). Maternal basking opportunity affects juvenile phenotype in a viviparous lizard. Functional Ecology, 14, 345–352.
Webb, J. K., Shine, R., & Christian, K. A. (2006). The adaptive significance of reptilian viviparity in the tropics: testing the maternal manipulation hypothesis. Evolution, 60, 115–122.
Acknowledgments
We would like to thank Félix González for his assistance in collecting lizards, and Ronnie Lendrum for linguistic advice. Funding was provided by the Spanish Ministry of Science (M.E.C.) as a project grant (ref. CGL2007-60187) to Florentino Braña and a fellowship to Tania Rodríguez-Díaz (ref. AP2005-4296) co-financed by the European Social Fund. The lizards used in this study were collected under licence from the environmental authorities of the Junta de Castilla y León and were released back into the wild at their places of capture after the experiments were completed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Díaz, T., Braña, F. Shift in Thermal Preferences of Female Oviparous Common Lizards During Egg Retention: Insights into the Evolution of Reptilian Viviparity. Evol Biol 38, 352–359 (2011). https://doi.org/10.1007/s11692-011-9122-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-011-9122-y