Abstract
Purpose
Plasmodium ovale is not usually the focus of most malaria research or intervention programmes and has lately been termed the neglected human malaria parasites. The parasite exists as two genetically distinct sympatric species namely P. ovale curtisi and P. ovale wallikeri but information on the distribution of P. ovale sub-species is lacking in Nigeria. The objective of this study, therefore, was aimed at characterizing the P. ovale sub-species in isolates from symptomatic individuals in North-central Nigeria.
Methods
Parasites were identified by light microscopy of Giemsa stained thick and thin blood films. Molecular characterization and confirmation of P. ovale sub-species were done by species-specific nested PCR and sequencing of the small subunit ribosomal RNA (SSUrRNA) gene.
Results
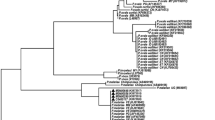
A total of 412 children were enrolled into this study of which 88.6% (n = 365) were positive for Plasmodium species by nested PCR and P. falciparum was predominant. Of the 365 isolates, 4 (1.1%) had P. ovale infections and of these, 3 (0.8%) were mixed species infections of P. ovale with P. falciparum. DNA sequence analysis confirmed that all the four P. ovale parasites were P. ovale curtisi as their sequences were 99–100% identical to previously published P. ovale curtisi sequences in the GenBank and they cluster with the P. ovale curtisi sequences by phylogeny.
Conclusion
Our findings demonstrate the occurrence of P. ovale curtisi in the study area. This has implications for public health and malaria elimination programmes, since they also serve as potential risk to travellers from malaria-free regions.




Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
References
Gething PW, Casey DC, Weiss DJ, Bisanzio D, Bhatt S, Cameron E, Battle KE, Dalrymple U, Rozier J, Rao PC, Kutz MJ, Barber RM, Huynh C, Shackelford KA, Coates MM, Nguyen G, Fraser MS, Kulikoff R, Wang H, Naghavi M, Smith DL, Murray CJ, Hay SI, Lim SS (2016) Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med 375(25):2435–2445. https://doi.org/10.1056/NEJMoa1606701
WHO (2019) World malaria report 2019. WHO, Geneva. https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 19 Mar 2020
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014) Malaria. Lancet 383(9918):723–735. https://doi.org/10.1016/S0140-6736(13)60024-0
WHO (2018) World Malaria Report 2018. WHO, Geneva. https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. Accessed 14 Dec
Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B (2008) Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 46(2):165–171. https://doi.org/10.1086/524888
Li P, Zhao Z, Xing H, Li W, Zhu X, Cao Y, Yang Z, Sattabongkot J, Yan G, Fan Q, Cui L (2016) Plasmodium malariae and Plasmodium ovale infections in the China-Myanmar border area. Malar J 15(1):557. https://doi.org/10.1186/s12936-016-1605-y
Roucher C, Rogier C, Sokhna C, Tall A, Trape JF (2014) A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE 9(2):e87169. https://doi.org/10.1371/journal.pone.0087169
Daniels RF, Deme AB, Gomis JF, Dieye B, Durfee K, Thwing JI, Fall FB, Ba M, Ndiop M, Badiane AS, Ndiaye YD, Wirth DF, Volkman SK, Ndiaye D (2017) Evidence of non-Plasmodium falciparum malaria infection in Kedougou, Senegal. Malar J 16(1):9. https://doi.org/10.1186/s12936-016-1661-3
Stephens JWW (1922) A new malaria parasite of man. Ann Trop Med Parasitol 16(1):383–388. https://doi.org/10.1080/00034983.1922.11684331
Collins WE, Jeffery GM (2005) Plasmodium ovale: parasite and disease. Clin Microbiol Rev 18(3):570–581. https://doi.org/10.1128/CMR.18.3.570-581.2005
Mueller I, Zimmerman PA, Reeder JC (2007) Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol 23(6):278–283. https://doi.org/10.1016/j.pt.2007.04.009
D’Abramo A, Gebremeskel Tekle S, Iannetta M, Scorzolini L, Oliva A, Paglia MG, Corpolongo A, Nicastri E (2018) Severe Plasmodium ovale malaria complicated by acute respiratory distress syndrome in a young Caucasian man. Malar J 17(1):139. https://doi.org/10.1186/s12936-018-2289-2
Lau YL, Lee WC, Tan LH, Kamarulzaman A, Syed Omar SF, Fong MY, Cheong FW, Mahmud R (2013) Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar J 12:389. https://doi.org/10.1186/1475-2875-12-389
Merrick CJ (2020) Hypnozoites in Plasmodium: do parasites parallel plants? Trends Parasitol S1471–4922(20):30299–30303. https://doi.org/10.1016/j.pt.2020.11.001
Calderaro A, Piccolo G, Gorrini C, Montecchini S, Rossi S, Medici MC, Chezzi C, Snounou G (2012) A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS ONE 7(10):e48033. https://doi.org/10.1371/journal.pone.0048033
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH (2007) A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77(6 Suppl):119–127. https://doi.org/10.4269/ajtmh.2007.77.119
Ajakaye OG, Ibukunoluwa MR (2020) Performance evaluation of a popular malaria RDT in Nigeria compared with microscopy. J Parasit Dis 44(1):122–125. https://doi.org/10.1007/s12639-019-01170-y
Berzosa P, Gonzalez V, Taravillo L, Mayor A, Romay-Barja M, Garcia L, Ncogo P, Riloha M, Benito A (2020) First evidence of the deletion in the pfhrp2 and pfhrp3 genes in Plasmodium falciparum from Equatorial Guinea. Malar J 19(1):99. https://doi.org/10.1186/s12936-020-03178-9
Kanwugu ON, Helegbe GK, Aryee PA, Abdul-Karim A, Anaba F, Ziblim Z, Amevi ED (2019) Prevalence of asymptomatic malaria among children in the tamale metropolis: how does the PfHRP2 CareStart RDT perform against microscopy? J Trop Med 2019:6457628. https://doi.org/10.1155/2019/6457628
Kojom LP, Singh V (2020) Prevalence of Plasmodium falciparum field isolates with deletions in histidine-rich protein 2 and 3 genes in context with sub-Saharan Africa and India: a systematic review and meta-analysis. Malar J 19(1):46. https://doi.org/10.1186/s12936-019-3090-6
Niyibizi JB, Gatera EK (2020) Diagnostic performance between histidine-rich protein 2 (HRP-2), a rapid malaria diagnostic test and microscopic-based staining techniques for diagnosis of malaria. J Trop Med 2020:5410263. https://doi.org/10.1155/2020/5410263
Ojurongbe O, Adegbosin OO, Taiwo SS, Alli OA, Olowe OA, Ojurongbe TA, Bolaji OS, Adeyeba OA (2013) Assessment of clinical diagnosis, microscopy, rapid diagnostic tests, and polymerase chain reaction in the diagnosis of Plasmodium falciparum in Nigeria. Malar Res Treat 2013:308069. https://doi.org/10.1155/2013/308069
Funwei R, Nderu D, Nguetse CN, Thomas BN, Falade CO, Velavan TP, Ojurongbe O (2019) Molecular surveillance of pfhrp2 and pfhrp3 genes deletion in Plasmodium falciparum isolates and the implications for rapid diagnostic tests in Nigeria. Acta Trop 196:121–125. https://doi.org/10.1016/j.actatropica.2019.05.016
Haanshuus CG, Chandy S, Manoharan A, Vivek R, Mathai D, Xena D, Singh A, Langeland N, Blomberg B, Vasanthan G, Sitaram U, Appasamy J, Nesaraj J, Henry A, Patil S, Alvarez-Uria G, Armstrong L, Morch K (2016) A high malaria prevalence identified by PCR among patients with acute undifferentiated fever in India. PLoS ONE 11(7):e0158816. https://doi.org/10.1371/journal.pone.0158816
Liew JW, Mahmud R, Tan LH, Lau YL (2016) Diagnosis of an imported Plasmodium ovale wallikeri infection in Malaysia. Malar J 15:8. https://doi.org/10.1186/s12936-015-1070-z
Proux S, Suwanarusk R, Barends M, Zwang J, Price RN, Leimanis M, Kiricharoen L, Laochan N, Russell B, Nosten F, Snounou G (2011) Considerations on the use of nucleic acid-based amplification for malaria parasite detection. Malar J 10:323. https://doi.org/10.1186/1475-2875-10-323
Roth JM, Korevaar DA, Leeflang MM, Mens PF (2016) Molecular malaria diagnostics: a systematic review and meta-analysis. Crit Rev Clin Lab Sci 53(2):87–105. https://doi.org/10.3109/10408363.2015.1084991
Waters AP, McCutchan TF (1989) Rapid, sensitive diagnosis of malaria based on ribosomal RNA. Lancet 1(8651):1343–1346. https://doi.org/10.1016/s0140-6736(89)92800-6
Oyedeji SI, Awobode HO, Monday GC, Kendjo E, Kremsner PG, Kun JF (2007) Comparison of PCR-based detection of Plasmodium falciparum infections based on single and multicopy genes. Malar J 6:112. https://doi.org/10.1186/1475-2875-6-112
Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA (1999) A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 60(4):687–692. https://doi.org/10.4269/ajtmh.1999.60.687
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN (1993) High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61(2):315–320. https://doi.org/10.1016/0166-6851(93)90077-b
Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, Proietti C, Bousema T, Ndounga M, Tanabe K, Ntege E, Culleton R, Sutherland CJ (2011) Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol 41(6):677–683. https://doi.org/10.1016/j.ijpara.2011.01.004
Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, Dolecek C, Hien TT, do Rosario VE, Arez AP, Pinto J, Michon P, Escalante AA, Nosten F, Burke M, Lee R, Blaze M, Otto TD, Barnwell JW, Pain A, Williams J, White NJ, Day NP, Snounou G, Lockhart PJ, Chiodini PL, Imwong M, Polley SD (2010) Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis 201(10):1544–1550. https://doi.org/10.1086/652240
Win TT, Lin K, Mizuno S, Zhou M, Liu Q, Ferreira MU, Tantular IS, Kojima S, Ishii A, Kawamoto F (2002) Wide distribution of Plasmodium ovale in Myanmar. Trop Med Int Health 7(3):231–239. https://doi.org/10.1046/j.1365-3156.2002.00857.x
Calderaro A, Piccolo G, Perandin F, Gorrini C, Peruzzi S, Zuelli C, Ricci L, Manca N, Dettori G, Chezzi C, Snounou G (2007) Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J Clin Microbiol 45(5):1624–1627. https://doi.org/10.1128/JCM.02316-06
Miller RH, Obuya CO, Wanja EW, Ogutu B, Waitumbi J, Luckhart S, Stewart VA (2015) Characterization of Plasmodium ovale curtisi and P. ovale wallikeri in Western Kenya utilizing a novel species-specific real-time PCR assay. PLoS Negl Trop Dis 9(1):e0003469. https://doi.org/10.1371/journal.pntd.0003469
Miyake H, Suwa S, Kimura M, Wataya Y (1997) A variant of Plasmodium ovale; analysis of its 18S ribosomal RNA gene sequence. Nucleic Acids Symp Ser 37:293–294
Tachibana M, Tsuboi T, Kaneko O, Khuntirat B, Torii M (2002) Two types of Plasmodium ovale defined by SSU rRNA have distinct sequences for ookinete surface proteins. Mol Biochem Parasitol 122(2):223–226. https://doi.org/10.1016/s0166-6851(02)00101-9
Li J, Wirtz RA, McConkey GA, Sattabongkot J, Waters AP, Rogers MJ, McCutchan TF (1995) Plasmodium: genus-conserved primers for species identification and quantitation. Exp Parasitol 81(2):182–190. https://doi.org/10.1006/expr.1995.1107
Fuehrer HP, Noedl H (2014) Recent advances in detection of Plasmodium ovale: implications of separation into the two species Plasmodium ovale wallikeri and Plasmodium ovale curtisi. J Clin Microbiol 52(2):387–391. https://doi.org/10.1128/JCM.02760-13
Ayogu EE, Ukwe CV, Nna EO (2016) Assessing the reliability of microscopy and rapid diagnostic tests in malaria diagnosis in areas with varying parasite density among older children and adult patients in Nigeria. J Postgrad Med 62(3):150–156. https://doi.org/10.4103/0022-3859.183167
Engelbrecht F, Togel E, Beck HP, Enwezor F, Oettli A, Felger I (2000) Analysis of Plasmodium falciparum infections in a village community in Northern Nigeria: determination of msp2 genotypes and parasite-specific IgG responses. Acta Trop 74(1):63–71. https://doi.org/10.1016/s0001-706x(99)00044-3
Yakubu B, Longdet IY, Jen TH, Davou DT, Obishakin E (2019) High-Complexity Plasmodium falciparum infections, North Central Nigeria, 2015–2018. Emerg Infect Dis 25(7):1330–1338. https://doi.org/10.3201/eid2507.181614
May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, Meyer CG (1999) High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg 61(2):339–343. https://doi.org/10.4269/ajtmh.1999.61.339
Oboh MA, Badiane AS, Ntadom G, Ndiaye YD, Diongue K, Diallo MA, Ndiaye D (2018) Molecular identification of Plasmodium species responsible for malaria reveals Plasmodium vivax isolates in Duffy negative individuals from southwestern Nigeria. Malar J 17(1):439. https://doi.org/10.1186/s12936-018-2588-7
Ugah UI, Alo MN, Owolabi JO, Okata-Nwali OD, Ekejindu IM, Ibeh N, Elom MO (2017) Evaluation of the utility value of three diagnostic methods in the detection of malaria parasites in endemic area. Malar J 16(1):189. https://doi.org/10.1186/s12936-017-1838-4
Oyedeji SI, Awobode HO, Bassi PU (2017) Molecular investigation of sub-microscopic and mixed Plasmodium species infection in North-Central Nigeria. Asian Pac J Trop Dis 7(4):220–224. https://doi.org/10.12980/APJTD.7.2017D6-415
Cao Y, Wang W, Liu Y, Cotter C, Zhou H, Zhu G, Tang J, Tang F, Lu F, Xu S, Gu Y, Zhang C, Li J, Cao J (2016) The increasing importance of Plasmodium ovale and Plasmodium malariae in a malaria elimination setting: an observational study of imported cases in Jiangsu Province, China, 2011–2014. Malar J 15(459):9. https://doi.org/10.1186/s12936-016-1504-2
Molineaux L, Gramiccia G (1980) The Garki Project: Research on the epidemiology and control of malaria in the Sudan savanna of West Africa. World Health Organization, Geneva, pp109–172. https://apps.who.int/iris/handle/10665/40316
Craig MH, Snow RW, le Sueur D (1999) A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today 15(3):105–111. https://doi.org/10.1016/s0169-4758(99)01396-4
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(Web Server issue):W465–W469. https://doi.org/10.1093/nar/gkn180
Groger M, Fischer HS, Veletzky L, Lalremruata A, Ramharter M (2017) A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria. Malar J 16(1):112. https://doi.org/10.1186/s12936-017-1759-2
Rojo-Marcos G, Rubio-Munoz JM, Angheben A, Jaureguiberry S, Garcia-Bujalance S, Tomasoni LR, Rodriguez-Valero N, Ruiz-Giardin JM, Salas-Coronas J, Cuadros-Gonzalez J, Garcia-Rodriguez M, Molina-Romero I, Lopez-Velez R, Gobbi F, Calderon-Moreno M, Martin-Echevarria E, Elia-Lopez M, Llovo-Taboada J (2018) Prospective comparative multi-centre study on imported Plasmodium ovale wallikeri and Plasmodium ovale curtisi infections. Malar J 17(1):399. https://doi.org/10.1186/s12936-018-2544-6
Nabarro LEB, Nolder D, Broderick C, Nadjm B, Smith V, Blaze M, Checkley AM, Chiodini PL, Sutherland CJ, Whitty CJM (2018) Geographical and temporal trends and seasonal relapse in Plasmodium ovale spp. and Plasmodium malariae infections imported to the UK between 1987 and 2015. BMC Med 16(1):218. https://doi.org/10.1186/s12916-018-1204-6
Win TT, Jalloh A, Tantular IS, Tsuboi T, Ferreira MU, Kimura M, Kawamoto F (2004) Molecular analysis of Plasmodium ovale variants. Emerg Infect Dis 10(7):1235–1240. https://doi.org/10.3201/eid1007.030411
Chu R, Zhang X, Xu S, Chen L, Tang J, Li Y, Chen J, Xuan Y, Zhu G, Cao J, Cheng Y (2018) Limited genetic diversity of N-terminal of merozoite surface protein-1 (MSP-1) in Plasmodium ovale curtisi and P. ovale wallikeri imported from Africa to China. Parasites Vectors 11(596):1–8. https://doi.org/10.1186/s13071-018-3174-0
Dame JB, McCutchan TF (1983) The four ribosomal DNA units of the malaria parasite Plasmodium berghei. Identification, restriction map, and copy number analysis. J Biol Chem 258(11):6984–6990. https://www.jbc.org/content/258/11/6984.long
Acknowledgements
We thank all the participants who volunteered to participate in this study. Special thanks to the staff of Dalhatu Araf Specialist Hospital Lafia, Nigeria for their support in facilitating the study.
Funding
No funding was obtained for the study.
Author information
Authors and Affiliations
Contributions
SIO, HOA and CA designed the study. SIO and PUB collected sample. SIO, OO performed laboratory studies. SIO, HOA, OO, CA and PUB analysed the data, drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
We declare that we have no competing interests.
Ethics Approval
Statement of approval included under Materials and Methods Section.
Consent to Participate
Statement included under Materials and Methods Section.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyedeji, S.I., Awobode, H.O., Ojurongbe, O. et al. Molecular Identification and Characterization of Plasmodium ovale curtisi in Field Isolates from Symptomatic Children in North-Central Nigeria. Acta Parasit. 66, 915–924 (2021). https://doi.org/10.1007/s11686-021-00350-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00350-2