Abstract
The tendency to avoid punishment, called behavioral inhibition system, is an essential aspect of motivational behavior. Behavioral inhibition system is related to negative affect, such as anxiety, depression and pain, but its neural basis has not yet been clarified. To clarify the association between individual variations in behavioral inhibition system and brain 5-HT2A receptor availability and specify which brain networks were involved in healthy male subjects, using [18F]altanserin positron emission tomography and resting-state functional magnetic resonance imaging. Behavioral inhibition system score negatively correlated with 5-HT2A receptor availability in anterior cingulate cortex. A statistical model indicated that the behavioral inhibition system score was associated with 5-HT2A receptor availability, which was mediated by the functional connectivity between anterior cingulate cortex and left middle frontal gyrus, both of which involved in the cognitive control of negative information processing. Individuals with high behavioral inhibition system displays low 5-HT2A receptor availability in anterior cingulate cortex and this cognitive control network links with prefrontal-cingulate integrity. These findings have implications for underlying the serotonergic basis of physiologies in aversion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fundamental features of complex behavior have long been discussed as being categorizable into the approach to rewards and the avoidance of punishments. These two systems can be applied to account for personality and motivation (Davidson, 1994; Gray, 1982; Higgins et al., 1994), positing that there are independent sensitivity in the respective systems. Gray provided a powerful theoretical framework that was rooted in behavioral psychology and neuroscience, called Reinforcement Sensitivity Theory (RST) (Gray, 1982). Gray proposes two systems together with an additional one: Behavioral Approach System (BAS), Behavioral Inhibition System (BIS), and Fight-Flight System (FFS) (see revised version of RST(Gray & McNaughton, 2000)). The BAS promotes behavior that leads to positive outcomes (reward and non-punishment) and is involved in the experience of positive emotions such as hope, elation, and happiness, while the BIS causes inhibition of behaviors that lead to negative outcomes (punishment and non-reward) and is involved in the experience of negative emotions such as fear, anxiety, frustration, and sadness. BAS corresponds to impulsivity, drug addiction, and attention deficit hyperactivity disorder; BIS is a self-reported sensitivity to punishment related to anxiety, depression, and pain, and FFS is fear at the psychological and psychiatric level (Bijttebier et al., 2009; Corr, 2002, 2004; Jensen et al., 2016).
In contrast to BAS, however, only a handful of studies have investigated the neural basis of BIS. The trait sensitivity to aversive events was associated with increased gray matter volume in amygdala and hippocampus (Barros-Loscertales et al., 2006; Cherbuin et al., 2008) and decreased volume in orbitofrontal cortex (OFC) and precuneus (Fuentes et al., 2012). BIS variability was also associated with individual differences in the neural activities of dorsal anterior cingulate cortex (ACC), OFC, striatum, amygdala, and hippocampus during anticipation of aversive events, such as monetary loss, measured by functional magnetic resonance imaging (fMRI) (Beaver et al., 2008; Kim et al., 2015; Simon et al., 2010). A resting-state fMRI (rs-fMRI) study similarly found that BIS correlated negatively with regional homogeneity in amygdala and hippocampus (Hahn et al., 2013).
Meanwhile, a number of neuroimaging studies have investigated the neural responses to aversive stimuli such as signal pain, punishment and monetary loss. The core regions of these aversive anticipations are found in ACC, anterior insula, OFC, and amygdala (De Martino et al., 2010; Eisenberger, 2012; Hayes & Northoff, 2011; Kringelbach & Rolls, 2004; Nitschke et al., 2006; Wrase et al., 2007). Congruent brain regions (ACC, OFC, amygdala) between aversive anticipation and individual variations in the sensitivity to aversive events leads to the notion that these regions are the hub for understanding the neural mechanisms of BIS.
According to Gray’s concept of BIS, harm avoidance is characterized by excessive anxiety and fear. Serotonin 2C(5-HT2C) receptors are linked to some of the adverse motivational effects corresponding to avoidance behaviors (Roberts et al., 2020), but 5-HT2A receptors have also been reported to be intimately involved in the modulation of negative emotions, such as anxiety, depression, and pain (Baldwin & Rudge, 1995; Sommer, 2009). For instance, higher pessimistic behavior in depressive patients was related to higher frontal 5-HT2A receptor binding as detected by positron emission tomography (PET) (Meyer et al., 2003). Harm avoidance and 5-HT2A receptor availability showed a negative correlation in the prefrontal cortex and left parietal cortex (Moresco et al., 2002), a positive correlation in the dorsal prefrontal cortex (Baeken et al., 2014), or no significant regional correlation (Soloff et al., 2010). Although 5-HT2A receptor agonists may experimentally increase impulsivity (Carli et al., 2006), the human [18F]altanserin PET study was unable to prove these relationships, in addition to a prior report (da Cunha-Bang et al., 2013; Frokjaer et al., 2008). Human PET studies have shown that 5-HT2A receptors are numerous and widely distributed in cortical regions (Savli et al., 2012), and it remains unclear whether individual variations in 5-HT2A receptor availability are involved in trait sensitivity to aversive events, that is, BIS.
The aim of this study was to elucidate the neural and molecular mechanisms associated with individual variations in BIS. In this regard, the relationships among BIS, the 5-HT2A receptor availability using PET and the brain functional connectivity measured by rs-fMRI were investigated. We first conducted a PET imaging study to explore which brain regions of 5-HT2A receptor availability correlated with BIS in healthy volunteers. Then, we analyzed rs-fMRI data to detect functional connectivity showing correlation with local 5-HT2A receptor availability and BIS. Finally, mediation analysis was conducted to elucidate the relationships among BIS, functional connectivity and the 5-HT2A receptor availability.
Materials and methods
Participants
Sixteen healthy right-handed male subjects (age: 23.3 ± 2.9 years, mean ± standard deviation) were recruited. Two subjects were excluded due to incomplete data collection, and the data of fourteen participants (23.4 ± 2.9 years) were analyzed. The demographic summary is shown in Table 1. All participants were free of current and past psychiatric or somatic disorders and had no history of drug abuse. Each participant completed psychological testing and underwent both rs-fMRI and PET scans. All participants provided written informed consent before participating in the study, which was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Psychological measurement
To test Gray’s original theory, Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) was developed by Torrubia (Torrubia et al., 2001). This scale indicates good reliability and validity, and accurately expresses the essence of Gray’s theory (e.g., extraversion and neuroticism in expected directions). All participants completed the Japanese version of SPSRQ (Takahashi & Shigemasu, 2008). SPSRQ is a 48-item self-report measure that consists of two subscales, representing sensitivity to reward (SR) to measure impulsivity, i.e., BAS, and sensitivity to punishment (SP) to measure anxiety, i.e., BIS. Each item is scored on a 4-point Likert scale (1 = disagree, 4 = agree). SR and SP scores with higher scores indicating greater impulsivity and sensitivity to punishment, respectively. Participants also completed the Beck Hopelessness Scale (BHS(Beck et al., 1974)) and State-Trait Anxiety Inventory (STAI (Spielberger et al., 1983)) to measure the levels of depressive hopelessness and anxiety, respectively.
PET acquisition and analysis
All subjects underwent a PET scan to measure regional 5-HT2A receptor availability. Although [18F]altanserin is a reversible and selective antagonist of the rat 5-HT2A receptor subtype (Riss et al., 2011), the changes in endogenous 5-HT binding do not directly influence the binding of [18F]altanserin (Kristiansen et al., 2005). A 90-min dynamic PET acquisition was performed after injection of [18F]altanserin (190 ± 5.4 MBq with molar activity of 167 ± 77 GBq/μmol). The scan protocol consisted of 33 frames (10 s × 6, 20 s × 3, 1 min × 6, 3 min × 4, and 5 min × 14 frames). All of the PET scans were performed on an Eminence SET-3000 GCT/X PET scanner (Shimadzu; Kyoto, Japan) with a head fixation device to minimize head movement. Each PET scan was preceded by a transmission scan for attenuation correction using a 137Cs source. All PET images were reconstructed with the filtered back-projection method (Gaussian filter, kernel 5 mm; reconstructed in-plane resolution was 7.5 mm in full width at half maximum; voxel size: 2 × 2 × 2.6 mm) corrected for attenuation, randoms and scatter.
During the scans, arterial blood samples were obtained manually 33 times after radioligand injection to obtain arterial input function (Ishii et al., 2017). Each blood sample was centrifuged to obtain plasma and blood cell fractions, and the concentrations of radioactivity in whole blood and plasma were measured (Ishii et al., 2017). The fractions of the parent compound and its radiometabolites in plasma were determined using high-performance liquid chromatography from 6 samples of each subject (Ishii et al., 2017).
All PET images were spatially normalized to the standard anatomic orientation. First, head motion during the scans was corrected on the emission images after correction of attenuation using µ-maps that were realigned to each frame of the emission images (Wardak et al., 2010). Second, T1-weighted MR images were coregistered to the corresponding mean PET images. Third, the MR images were spatially normalized and segmented into gray matter, white matter, and cerebrospinal fluid using SPM8 (Wellcome Institute of Neurology, University College of London, UK). Finally, all PET images were spatially normalized to the standard anatomic orientation (Montreal Neurological Institute (MNI) 152 standard space; Montreal Neurological Institute; Montreal, QC, Canada) based on the transformation of the MR images.
Because the Logan analysis provided a good compromise between validity, sensitivity, and reliability of implementation (Price et al., 2001), the PET data were analyzed by Logan graphical method (Logan et al., 1990), which was applied across the 12- to 90-min integration intervals, and regional total distribution volume (VT) values were obtained. We used the cerebellum as reference brain region and estimated the nondisplaceable distribution volume (VND). 5-HT2A receptor availability was determined as binding potential (BPP) that was derived from the equation: BPP = VT–VND (Innis et al., 2007). All kinetic analyses were performed using PMOD (version 3.6, PMOD Technologies Ltd., Zurich, Switzerland).
Region-of-interest analysis
ACC, OFC and amygdala, which are involved in the sensitivity to aversive events (Barros-Loscertales et al., 2006; Beaver et al., 2008; Cherbuin et al., 2008; Eisenberger, 2012; Fuentes et al., 2012), were applied to ROI analyses. These brain regions were extracted by the Harvard–Oxford atlas using the CONN toolbox (version 17e, http://www.nitrc.org/projects/conn), averaged over right and left. Subsequently, ACC was divided into four segregated subregions, namely, subgenual ACC (sgACC), pregenual ACC (pgACC), anterior midcingulate cortex (aMCC) and posterior midcingulate cortex (pMCC) (Yeung et al., 2004). Subgenual ACC was substituted by subcallosal cortex in the atlas due to small volume (0.056 mm3). The volumes of each ACC subregion were 9.2 mm3 in subcallosal cortex, 6.5 mm3 in pgACC, 7.6 mm3 in aMCC, and 6.7 mm3 in pMCC. The volumes of the OFC and amygdala were 25.3 mm3 and 5.4 mm3, respectively.
Resting-state fMRI acquisition and analysis
Each subject underwent a 6.8-min rs-fMRI scan, performed with a Magnetom Verio 3.0 T MRI scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head coil. During scanning, subjects were instructed to relax with their eyes open while gazing at a fixation cross. A single session acquired 3.8-mm thick, no gap, interleaved axial 33 slices (in-plane resolution: 3.75 × 3.75 mm) with a 30-degree angle relative to the AC-PC axis, using a T2*-sensitive single-shot EPI sequence with the following parameters: TR = 2000 ms, TE = 25 ms, flip angle = 90 degrees, matrix = 64 × 64. A high-resolution T1-weighted anatomical image using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (176 sagittal slices, resolution = 0.49 × 0.49 × 1.00 mm, no gap, TR = 2300 ms, TE = 1.95 ms, flip angle = 9 degrees, matrix = 512 × 512) was acquired for anatomical reference.
Data processing was performed using the CONN toolbox and SPM12 (Wellcome Institute of Neurology, University College of London, UK) working on Matlab version 8.4 (MathWorks, MA, USA). The first four volumes were discarded from analysis to account for magnetization saturation effects. Preprocessing comprised: 1) realignment and unwarping, 2) slice timing correction, 3) segmentation and normalization, 4) smoothing with a Gaussian kernel of 4 mm. To eliminate correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using Artifact Detection Tools (ART), which includes the CONN toolbox. For each subject, we treated images as outliers if composite movement from a preceding image exceeded 0.2 mm, or if the global mean intensity was over 3 SDs from the mean image intensity for the entire resting scan. Based on the previous report(Yan et al., 2013), after removing outlier images, one subject whose total scan time of less than 3 min was excluded from the subsequent analyses.
After preprocessing, we conducted de-noising as follows: 1) linear regression of noise sources from white matter and cerebrospinal fluid by CompCor (component-based noise correction method) and from outliers by ART and from Friston 24 head motion parameter, 2) band-pass filtering of 0.009 – 0.1 Hz was used to pass the low frequency fluctuations of interest, 3) quadratic trends were removed. Global signal regression was not used to avoid potential false anticorrelations.
Image analyses
Association between SP and 5-HT2A receptor availability
To test for the contribution of 5-HT2A receptor availability to SP, we first conducted Spearman’s rank test between SP and the BPP value of each ROI using GraphPad Prism (version 7, GraphPad Software, CA, USA). P-value less than 0.05 with false discovery rate (FDR) correction for multiple comparisons was considered significant.
Relationship between 5-HT2A receptor availability and functional connectivity
Regions of 5HT2A receptor availability that correlated with SP were subsequently investigated by seed-based functional connectivity analysis modeling the BPP values in the analogous regions, utilizing the CONN toolbox. This procedure may explore the functional connectivities that correlate with 5-HT2A receptor availability of SP-related ROIs. The threshold was defined as a cluster-level threshold of p < 0.05, FDR-corrected with voxel-level threshold of p < 0.001, uncorrected for multiple comparisons. All reported coordinates were of MNI standard space.
Functional connectivity related to SP
The correlation coefficient for each specified functional connectivity was extracted. Spearman’s rank test was performed between each extracted correlation coefficient and SP. P-value less than 0.05 was considered significant.
Mediation analysis
Finally, for each functional connectivity that was significantly related to both 5-HT2A receptor availability and SP, we performed mediation analysis to test whether functional connectivity might be involved in the link between SP and 5-HT2A receptor availability. The correlation coefficient of functional connectivity was included as a mediator. INDIRECT macro (Preacher & Hayes, 2008) with SPSS (version 24, IBM, NY, USA) was used. Bias-corrected and accelerated 95% confidence intervals based on 10,000 bootstrap sampling were used to assess significance.
Results
Behavioral findings
The median SP and SR scores were 61 (interquartile range, 55–67.5) and 51 (interquartile range, 44–59), respectively (Table 1). The median score of BHS was 6 (interquartile range, 4.5–10.5) and STAI was 41 (interquartile range, 34–54.5). The SP scores correlated positively with the BHS (rs = 0.86, p < 0.0005, Spearman’s rank test) and at a marginally significant level with STAI (rs = 0.52, p = 0.07).
Regional 5-HT2A receptor availability measured by [18F]altanserin PET
Figure 1 shows the 5-HT2A receptor availability values (BPP) in ACC, OFC and amygdala which were selected from the premise that these regions were associated with aversive anticipation. Higher BPP was measured in the ACC and OFC, while low BPP was observed in the amygdala. The BPP values in ACC (1.535 [1.376 to 1.773]), OFC (1.503 [1.41 to 1.633]) and amygdala (0.676 [0.581 to 0.762]) were comparable to that of healthy subjects in previous report (Savli et al., 2012).
Association between behavioral inhibition system and 5-HT2A receptor availability
The SP score was negatively correlated with the BPP value of ACC (rs = -0.66, p = 0.016, Spearman’s rank test with FDR correction). No correlations were found in OFC or amygdala (rs = -0.47 and rs = -0.57, respectively, both p > 0.05 with FDR correction). There was no significant correlation between the SR score and BPP values (ACC, rs = 0.28, p = 0.35; OFC, rs = 0.02, p = 0.96; amygdala, rs = 0.11, p = 0.73).
We further examined the correlations between SP and the BPP values in the functional subdivisions of ACC. There was no difference in the BPP values among subdivisions of ACC (F(3, 48) = 1.06, p = 0.375, One-way ANOVA). SP scores correlated negatively with the BPP values in pgACC, aMCC and pMCC but not in subcallosal cortex (pgACC, rs = -0.59, p = 0.037; aMCC, rs = -0.60, p = 0.034; pMCC, rs = -0.66, p = 0.017; subcallosal cortex, rs = -0.46, p = 0.117, Spearman’s rank test with FDR correction; Fig. 2).
a) Mean parametric image of 5-HT2A receptor binding of [18F]altanserin PET, shown in sagittal view. b) Subdivisions of ACC, overlaid on sagittal T1 MRI template. c) Plot graph of sensitivity to punishment (SP) score and regional 5-HT2A receptor binding potentials (BPP). SP was negatively correlated with 5-HT2A receptor BPP in pgACC, aMCC and pMCC, whereas no such association was detected in subcallosal region (*false discovery rate corrected p < 0.05). Spearman ‘s rank test was used. pgACC, pregenual anterior cingulate cortex; aMCC, anterior midcingulate cortex; pMCC, posterior midcingulate cortex
Association between 5-HT2A receptor availability and functional connectivity
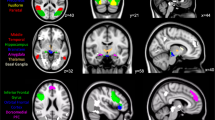
Seed-based functional connectivity analyses were performed for above three ACC subregions (Fig. 3, Table 2) to explore functional connectivity that correlated with the local BPP value. The BPP values in pgACC were negatively correlated with the functional connectivity between pgACC and clusters in left lateral occipital cortex and right lingual gyrus (Fig. 3a). The BPP values in aMCC were positively correlated with the functional connectivity between aMCC and left middle frontal gyrus (MFG) (Fig. 3b). The BPP values in pMCC were positively correlated with the functional connectivity between pMCC and clusters in right inferior frontal gyrus, left precentral gyrus, left supramarginal gyrus and left angular gyrus (Fig. 3c).
Surface rendered images of functional connectivity associated with 5-HT2A receptor binding potentials in each anterior cingulate cortex subregion. a) 5-HT2A receptor binding potentials in the pgACC were negatively correlated with the functional connectivity of the pregenual anterior cingulate cortex. b) 5-HT2A receptor binding potentials in the anterior midcingulate cortex were positively correlated with the functional connectivity of the anterior midcingulate cortex. c) 5-HT2A receptor binding potentials in the posterior midcingulate cortex were positively correlated with the functional connectivity of the posterior midcingulate cortex. Shown clusters remained after a threshold of cluster-level p < 0.05 false discovery rate corrected and voxel-level p < 0.001 uncorrected for multiple comparisons. Clusters were surface-rendered onto a brain template. Color bar represents T-value; negative correlations as blue-purple, positive correlations as red-yellow
Functional connectivity related to behavioral inhibition system
Correlation analyses between these specific functional connectivities and SP were carried out. SP was negatively correlated with the functional connectivity between aMCC and left MFG (rs = -0.67, p = 0.014, Spearman’s rank test; Table 3).
Mediation analysis
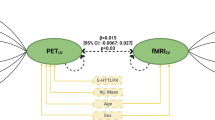
Finally, we examined whether functional connectivity between aMCC and left MFC serve as a potential mediator of the link between SP scores and 5-HT2A receptor availability. We tested two possible models: 1) 5-HT2A receptor availability affects functional connectivity, which in turn affects SP; 2) SP affects functional connectivity, which in turn affects 5-HT2A receptor availability. Mediation analyses supported the latter model, indicating that the total indirect effect of SP scores on the BPP values via functional connectivity was significant (Bias-corrected and accelerated 95% confidence intervals: -0.044 to -0.007; p < 0.05). In sum, these results imply that the functional connectivity between aMCC and left MFG serves as an important role in linking BIS with the 5-HT2A receptor availability.
Discussion
This study investigated whether the individual variations in 5-HT2A receptor availability contributes to BIS and which brain networks were specifically involved. BIS correlated negatively with 5-HT2A receptor availability in ACC, and the association between BIS and 5-HT2A receptor availability was accounted for by the functional connectivity between aMCC and left MFG.
Our findings indicate the role of 5-HT2A receptor-mediated serotonergic neurotransmission in ACC, as was previously linked with BIS personality and aversive anticipation. Specifically, high BIS individuals showed reduced levels of serotonin 5-HT2A receptor availability in ACC. The 5-HT2A receptor is known to be related to psychiatric symptoms, such as anxiety and depression, as well as hallucinations in schizophrenia (Quednow et al., 2009) and Parkinson’s disease (Ballanger et al., 2010). In recent genetic studies, polymorphism of the 5-HT2A gene has been frequently reported in depression and schizophrenia (Gu et al., 2013; Tan et al., 2014; Zhao et al., 2014). For example, single nucleotide polymorphism of 5-HT2A gene was associated with pathological gambling and suicide in depressed patients (Arias et al., 2001; Wilson et al., 2013). Furthermore, in PET studies, while suicide victims had a high density of 5-HT2A receptors in prefrontal cortex (Du et al., 2001), treatment-resistant depressed patients displayed lower 5-HT2A receptor binding in dorsal prefrontal cortex and ACC (Baeken et al., 2012). These contradictory findings represent the activation and the inhibition of impulsivity (Fineberg et al., 2010), and the current finding supports to the latter. Animal experiments have also shown that blockage of the 5-HT2A receptor in medial prefrontal cortex suppressed impulsive behavior (Fink et al., 2015); human experiments have shown that 5-HT2A agonists facilitate punishment learning (Kanen et al., 2021). A recent review suggested that 5-HT2A signaling is associated with cognitive flexibility (Carhart-Harris & Nutt, 2017). Although the causal relationship between 5-HT2A receptor availability and BIS still needs to be verified, the present results of mediation analyses indicate that a high level of BIS, which positively correlates with depressive hopelessness, causes low 5-HT2A receptor availability in aMCC via the functional connectivity of aMCC. This may reflect the inhibitory control and cognitive flexibility associated with some aspects of depressive symptoms, which may lead to downregulation of the 5-HT2A receptor.
This study newly identified the functional connectivity associated with 5-HT2A receptor availability in ACC subregions (pgACC, aMCC, pMCC), and the mediation test revealed that aMCC-MFG functional connectivity contributed to the link between BIS and 5-HT2A receptor availability. ACC has been consistently linked with cognitive function, emotion processing, and the autonomic nervous system (Bush et al., 2000; Critchley et al., 2001), which is divided into 4 subregions (Vogt et al., 2003). The pgACC, the ventral part of ACC, is involved in assessing emotional and motivational information. The pMCC and aMCC are part of the dorsal ACC, mainly involved in cognitive controls. The negative correlation between the BPP of the pgACC and the functional connectivity between the pgACC and left lateral occipital cortex/right lingual gyrus might be associated with the psychedelic state, although it is not possible to draw any conclusions from the current results. Lysergic acid diethylamide (LSD), a non-selective 5-HT2A receptor agonist and well-known hallucinogenic agent, was administered to healthy subjects who underwent fMRI scanning, which revealed an association with the activation of the primary visual cortex, which was correlated with the degree of visual hallucination, represented as a ‘psychedelic state’ (Carhart-Harris et al., 2016).
The aMCC represents a hub where information about punishment and negative feedback, such as pain, is monitored, triggering control signals and/or selective attention generated in dorsolateral prefrontal cortex (DLPFC) (MacDonald et al., 2000; Miller & Cohen, 2001; Shackman et al., 2011; Walsh et al., 2011; Yeung et al., 2004). Other studies have also shown that aMCC is anatomically connected with DLPFC in monkeys (Morecraft & Tanji, 2009), and that the functional connectivity between these two regions is correlated with working memory demand according to task-based fMRI (Osaka et al., 2004). A recent study with multi-voxel pattern analysis supports the fact that the middle frontal gyri appear to be primarily predictive of the subjective experience of fear (Taschereau-Dumouchel et al., 2020). Consistent with these previous studies, our finding of the functional connectivity between aMCC and left MFG, a part of DLPFC, possibly reflects the inhibitory control and cognitive flexibility associated with negative information processing, and in particular, serves a mechanistic role in linking BIS and 5-HT2A receptor–mediated serotonergic neurotransmission.
It is puzzling that the direction of the path was found to be from the psychological trait to the molecular system, not vice versa. The mediation test taps on the mathematical linkage rather than on a biological one. 5-HT2A receptor–mediated stimulation by physiological and acute or chronic pharmacological manner may exhibit differently in the brain function. To further clarify this question, acute and chronic 5-HT2A receptor intervention may alter both BIS and the functional connectivity between aMCC and left MFG, which shall be left for the future investigations.
There are several limitations to this study. The first is that our sample size is comparatively small. Although we have set a stringent statistical threshold, future study with a larger sample size will be required to replicate the current findings. Second, females were not included in the present study. As estrogen promotes 5-HT synthesis and menstrual cycle, it influences 5-HT2A receptor binding in women (Wihlbäck et al., 2004), thereby we exclusively included male subjects in the current study. Considering that emotional reactions differ between genders, it may be interesting to explore the similarities and differences between male and female subjects in the future. In addition, the enrolled subjects were all Japanese. Previous behavioral studies have indicated that Japanese are motivated more by negative feedbacks than by positive ones (Diener et al., 2003; Heine et al., 2001); thus, our results might be biased in this regard. Lastly, functional connectivity only accounts for a linear association between two brain regions. Whole brain networks and anatomical connectivities were not examined in the present study and should be addressed in the future studies.
Conclusions
In summary, this multimodal neuroimaging study provides novel evidence of the relationship between the behavioral inhibition and the 5-HT2A receptor–mediated serotonergic function, which is mediated by the functional connectivity between aMCC and left MFG, known as a cognitive control network. The link obtained in the current study may be tested by interventional studies using drugs which modulate 5-HT2A receptor function to elucidate biological causal relationships. From the basis from the current findings, the symptoms related with behavioral inhibition of patients with anxiety, depression, and pain disorder may benefit from medications associated with 5-HT2A receptor function.
Data availability
Not applicable.
References
Arias, B., Gasto, C., Catalan, R., Gutierrez, B., Pintor, L., & Fananas, L. (2001). The 5-HT(2A) receptor gene 102T/C polymorphism is associated with suicidal behavior in depressed patients. American Journal of Medical Genetics, 105(8), 801–804. https://doi.org/10.1002/ajmg.10099
Baeken, C., De Raedt, R., & Bossuyt, A. (2012). Is treatment-resistance in unipolar melancholic depression characterized by decreased serotonin(2)A receptors in the dorsal prefrontal - anterior cingulate cortex? Neuropharmacology, 62(1), 340–346. https://doi.org/10.1016/j.neuropharm.2011.07.043
Baeken, C., Bossuyt, A., & De Raedt, R. (2014). Dorsal prefrontal cortical serotonin 2A receptor binding indices are differentially related to individual scores on harm avoidance. Psychiatry Research, 221(2), 162–168. https://doi.org/10.1016/j.pscychresns.2013.12.005
Baldwin, D., & Rudge, S. (1995). The role of serotonin in depression and anxiety. International Clinical Psychopharmacology, 9(Suppl 4), 41–45. https://doi.org/10.1097/00004850-199501004-00006
Ballanger, B., Strafella, A. P., van Eimeren, T., Zurowski, M., Rusjan, P. M., Houle, S., & Fox, S. H. (2010). Serotonin 2A receptors and visual hallucinations in Parkinson disease. Archives of Neurology, 67(4), 416–421. https://doi.org/10.1001/archneurol.2010.35
Barros-Loscertales, A., Meseguer, V., Sanjuan, A., Belloch, V., Parcet, M. A., Torrubia, R., & Avila, C. (2006). Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. NeuroImage, 33(3), 1011–1015. https://doi.org/10.1016/j.neuroimage.2006.07.025
Beaver, J. D., Lawrence, A. D., Passamonti, L., & Calder, A. J. (2008). Appetitive motivation predicts the neural response to facial signals of aggression. Journal of Neuroscience, 28(11), 2719–2725. https://doi.org/10.1523/JNEUROSCI.0033-08.2008
Beck, A. T., Weissman, A., Lester, D., & Trexler, L. (1974). The measurement of pessimism: The hopelessness scale. Journal of Consulting and Clinical Psychology, 42(6), 861–865. https://doi.org/10.1037/h0037562
Bijttebier, P., Beck, I., Claes, L., & Vandereycken, W. (2009). Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clinical Psychology Review, 29(5), 421–430. https://doi.org/10.1016/j.cpr.2009.04.002
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. https://doi.org/10.1016/s1364-6613(00)01483-2
Carhart-Harris, R. L., & Nutt, D. J. (2017). Serotonin and brain function: A tale of two receptors. Journal of Psychopharmacology, 31(9), 1091–1120. https://doi.org/10.1177/0269881117725915
Carhart-Harris, R. L., Muthukumaraswamy, S., Roseman, L., Kaelen, M., Droog, W., Murphy, K., … Nutt, D. J. (2016). Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proceedings of the National Academy of Sciences of the United States of America, 113(17), 4853–4858.https://doi.org/10.1073/pnas.1518377113
Carli, M., Baviera, M., Invernizzi, R. W., & Balducci, C. (2006). Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology, 31(4), 757–767. https://doi.org/10.1038/sj.npp.1300893
Cherbuin, N., Windsor, T. D., Anstey, K. J., Maller, J. J., Meslin, C., & Sachdev, P. S. (2008). Hippocampal volume is positively associated with behavioural inhibition (BIS) in a large community-based sample of mid-life adults: The PATH through life study. Social Cognitive and Affective Neuroscience, 3(3), 262–269. https://doi.org/10.1093/scan/nsn018
Cloninger, C. R. (1987). A systematic method for clinical description and classification of personality variants. A Proposal. Archives of General Psychiatry, 44(6), 573–588. https://doi.org/10.1001/archpsyc.1987.01800180093014
Corr, P. J. (2002). J A Gray’s reinforcement sensitivity theory: tests of the joint subsystems hypothesis of anxiety and impulsivity. Personality and Individual Differences, 33(4), 511–532.
Corr, P. J. (2004). Reinforcement sensitivity theory and personality. Neuroscience and Biobehavioral Reviews, 28(3), 317–332. https://doi.org/10.1016/j.neubiorev.2004.01.005
Critchley, H. D., Mathias, C. J., & Dolan, R. J. (2001). Neuroanatomical basis for first- and second-order representations of bodily states. Nature Neuroscience, 4(2), 207–212. https://doi.org/10.1038/84048
da Cunha-Bang, S., Stenbaek, D. S., Holst, K., Licht, C. L., Jensen, P. S., Frokjaer, V. G., … Knudsen, G. M. (2013). Trait aggression and trait impulsivity are not related to frontal cortex 5-HT2A receptor binding in healthy individuals. Psychiatry Research, 212(2), 125–131. https://doi.org/10.1016/j.pscychresns.2012.09.007
Davidson, R. J. (1994). Cerebral Asymmetry, Emotion, and Affective Style. In R. J. Davidson & K. Hugdahl (Eds.), Brain Asymmetry (pp. 361–387). MIT Press.
De Martino, B., Camerer, C. F., & Adolphs, R. (2010). Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3788–3792. https://doi.org/10.1073/pnas.0910230107
Diener, E., Oishi, S., & Lucas, R. E. (2003). Personality, culture, and subjective well-being: Emotional and cognitive evaluations of life. Annual Review of Psychology, 54, 403–425. https://doi.org/10.1146/annurev.psych.54.101601.145056
Du, L., Faludi, G., Palkovits, M., Bakish, D., & Hrdina, P. D. (2001). Serotonergic genes and suicidality. Crisis, 22(2), 54–60. https://doi.org/10.1027//0227-5910.22.2.54
Eisenberger, N. I. (2012). The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience, 13(6), 421–434. https://doi.org/10.1038/nrn3231
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., … Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology, 35(3), 591–604.https://doi.org/10.1038/npp.2009.185
Fink, L. H., Anastasio, N. C., Fox, R. G., Rice, K. C., Moeller, F. G., & Cunningham, K. A. (2015). Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacology, 40(8), 1957–1968. https://doi.org/10.1038/npp.2015.46
Frokjaer, V. G., Mortensen, E. L., Nielsen, F. A., Haugbol, S., Pinborg, L. H., Adams, K. H., … Knudsen, G. M. (2008). Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry, 63(6), 569–576.https://doi.org/10.1016/j.biopsych.2007.07.009
Fuentes, P., Barros-Loscertales, A., Bustamante, J. C., Rosell, P., Costumero, V., & Avila, C. (2012). Individual differences in the Behavioral Inhibition System are associated with orbitofrontal cortex and precuneus gray matter volume. Cognitive, Affective, & Behavioral Neuroscience, 12(3), 491–498. https://doi.org/10.3758/s13415-012-0099-5
Gray, J. A. (1982). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford University Press.
Gray, J. A., & McNaughton, N. (2000). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system (2 ed. Vol. 33). New York: Oxford University Press.
Gu, L., Long, J., Yan, Y., Chen, Q., Pan, R., Xie, X., … Su, L. (2013). HTR2A-1438A/G polymorphism influences the risk of schizophrenia but not bipolar disorder or major depressive disorder: a meta-analysis. Journal of Neuroscience Research, 91(5), 623–633.https://doi.org/10.1002/jnr.23180
Hahn, T., Dresler, T., Pyka, M., Notebaert, K., & Fallgatter, A. J. (2013). Local synchronization of resting-state dynamics encodes Gray’s trait Anxiety. PLoS ONE, 8(3), e58336. https://doi.org/10.1371/journal.pone.0058336
Hayes, D. J., & Northoff, G. (2011). Identifying a network of brain regions involved in aversion-related processing: A cross-species translational investigation. Frontiers in Integrative Neuroscience, 5, 49. https://doi.org/10.3389/fnint.2011.00049
Heine, S. J., Lehman, D. R., Ide, E., Leung, C., Kitayama, S., Takata, T., & Matsumoto, H. (2001). Divergent consequences of success and failure in japan and north america: An investigation of self-improving motivations and malleable selves. Journal of Personality and Social Psychology, 81(4), 599–615.
Higgins, E. T., Roney, C. J., Crowe, E., & Hymes, C. (1994). Ideal versus ought predilections for approach and avoidance: Distinct self-regulatory systems. Journal of Personality and Social Psychology, 66(2), 276–286. https://doi.org/10.1037//0022-3514.66.2.276
Innis, R. B., Cunningham, V. J., Delforge, J., Fujita, M., Gjedde, A., Gunn, R. N., … Carson, R. E. (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism, 27(9), 1533–1539.https://doi.org/10.1038/sj.jcbfm.9600493
Ishii, T., Kimura, Y., Ichise, M., Takahata, K., Kitamura, S., Moriguchi, S., … Suhara, T. (2017). Anatomical relationships between serotonin 5-HT2A and dopamine D2 receptors in living human brain. PLoS One, 12(12), e0189318.https://doi.org/10.1371/journal.pone.0189318
Jensen, M. P., Ehde, D. M., & Day, M. A. (2016). The Behavioral Activation and Inhibition Systems: Implications for Understanding and Treating Chronic Pain. The Journal of Pain, 17(5), 529 e521–529 e518. https://doi.org/10.1016/j.jpain.2016.02.001
Kanen, J. W., Luo, Q., Kandroodi, M. R., Cardinal, R. N., Robbins, T. W., Carhart-Harris, R. L., & den Ouden, H. E. M. (2021). Effect of lysergic acid diethylamide (LSD) on reinforcement learning in humans. bioRxiv. Retrieved from https://doi.org/10.1101/2020.12.04.412189
Kim, S. H., Yoon, H., Kim, H., & Hamann, S. (2015). Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning. Social Cognitive and Affective Neurosciences, 10(9), 1219–1227. https://doi.org/10.1093/scan/nsv007
Kringelbach, M. L., & Rolls, E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5), 341–372.
Kristiansen, H., Elfving, B., Plenge, P., Pinborg, L. H., Gillings, N., & Knudsen, G. M. (2005). Binding characteristics of the 5-HT2A receptor antagonists altanserin and MDL 100907. Synapse, 58(4), 249–257. https://doi.org/10.1002/syn.20205
Logan, J., Fowler, J. S., Volkow, N. D., Wolf, A. P., Dewey, S. L., Schlyer, D. J., … et al. (1990). Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. Journal of Cerebral Blood Flow and Metabolism, 10(5), 740–747.https://doi.org/10.1038/jcbfm.1990.127
MacDonald, A. W., 3rd., Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. https://doi.org/10.1126/science.288.5472.1835
Meyer, J. H., McMain, S., Kennedy, S. H., Korman, L., Brown, G. M., DaSilva, J. N., … Links, P. (2003). Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. American Journal of Psychiatry, 160(1), 90–99.https://doi.org/10.1176/appi.ajp.160.1.90
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Morecraft, R. J., & Tanji, J. (2009). Cingulofrontal Interactions and the Cingulate Motor Areas. In V. A. Vogt (Ed.), Cingulate Neurobiology and Disease (pp. 114–144). Oxford University Press.
Moresco, F. M., Dieci, M., Vita, A., Messa, C., Gobbo, C., Galli, L., … Fazio, F. (2002). In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. Neuroimage, 17(3), 1470–1478.https://doi.org/10.1006/nimg.2002.1239
Nitschke, J. B., Sarinopoulos, I., Mackiewicz, K. L., Schaefer, H. S., & Davidson, R. J. (2006). Functional neuroanatomy of aversion and its anticipation. NeuroImage, 29(1), 106–116. https://doi.org/10.1016/j.neuroimage.2005.06.068
Osaka, N., Osaka, M., Kondo, H., Morishita, M., Fukuyama, H., & Shibasaki, H. (2004). The neural basis of executive function in working memory: An fMRI study based on individual differences. NeuroImage, 21(2), 623–631. https://doi.org/10.1016/j.neuroimage.2003.09.069
Preacher, K. J., & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. https://doi.org/10.3758/brm.40.3.879
Price, J. C., Lopresti, B. J., Meltzer, C. C., Smith, G. S., Mason, N. S., Huang, Y., … Mathis, C. A. (2001). Analyses of [(18)F]altanserin bolus injection PET data. II: consideration of radiolabeled metabolites in humans. Synapse, 41(1), 11–21. https://doi.org/10.1002/syn.1055
Quednow, B. B., Geyer, M. A., & Halberstadt, A. L. (2009). Serotonin and Schizophrenia. In P. M. hristian & L. J. Barry (Eds.), Handbook of the Behavioral Neurobiology of Serotonin. (2 ed., Vol. 21, pp. 585–620). Academic Press.
Riss, P. J., Hong, Y. T., Williamson, D., Caprioli, D., Sitnikov, S., Ferrari, V., … Aigbirhio, F. I. (2011). Validation and quantification of [18F]altanserin binding in the rat brain using blood input and reference tissue modeling. Journal of Cerebral Blood Flow and Metabolism, 31(12), 2334–2342.https://doi.org/10.1038/jcbfm.2011.94
Roberts, C., Sahakian, B. J., & Robbins, T. W. (2020). Psychological mechanisms and functions of 5-HT and SSRIs in potential therapeutic change: Lessons from the serotonergic modulation of action selection, learning, affect, and social cognition. Neuroscience and Biobehavioral Reviews, 119, 138–167. https://doi.org/10.1016/j.neubiorev.2020.09.001
Savli, M., Bauer, A., Mitterhauser, M., Ding, Y. S., Hahn, A., Kroll, T., … Lanzenberger, R. (2012). Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage, 63(1), 447–459.https://doi.org/10.1016/j.neuroimage.2012.07.001
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. https://doi.org/10.1038/nrn2994
Simon, J. J., Walther, S., Fiebach, C. J., Friederich, H. C., Stippich, C., Weisbrod, M., & Kaiser, S. (2010). Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage, 49(2), 1868–1874. https://doi.org/10.1016/j.neuroimage.2009.09.016
Soloff, P. H., Price, J. C., Mason, N. S., Becker, C., & Meltzer, C. C. (2010). Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Research, 181(1), 77–84. https://doi.org/10.1016/j.pscychresns.2009.08.007
Sommer, C. (2009). Serotonin in Pain and Pain Control. In P. M. Christian & L. J. Barry (Eds.), Handbook of the Behavioral Neurobiology of Serotonin (2nd ed., Vol. 21, pp. 457–471). Academic Press.
Spielberger, C. D., Gorsuch, R. L., Lushene, R. E., Vagg, P. R., & Jacob, G. A. (1983). Manual for the state-trait anxiety inventory. Consulting Psychologist Press.
Takahashi, Y., & Shigemasu, K. (2008). Comparison of three scales measuring individual differences in sensitivity to punishment and reward. The Japanese Journal of Personality, 17(1), 72–81.
Tan, J., Chen, S., Su, L., Long, J., Xie, J., Shen, T., … Gu, L. (2014). Association of the T102C polymorphism in the HTR2A gene with major depressive disorder, bipolar disorder, and schizophrenia. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetic, 165B(5), 438–455.https://doi.org/10.1002/ajmg.b.32248
Taschereau-Dumouchel, V., Kawato, M., & Lau, H. (2020). Multivoxel pattern analysis reveals dissociations between subjective fear and its physiological correlates. Molecular Psychiatry, 25(10), 2342–2354. https://doi.org/10.1038/s41380-019-0520-3
Torrubia, R., Ávila, C., Moltó, J., & Caseras, X. (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862.
Vogt, B. A., Berger, G. R., & Derbyshire, S. W. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience, 18(11), 3134–3144. https://doi.org/10.1111/j.1460-9568.2003.03034.x
Walsh, B. J., Buonocore, M. H., Carter, C. S., & Mangun, G. R. (2011). Integrating conflict detection and attentional control mechanisms. Journal of Cognitive Neuroscience, 23(9), 2211–2221. https://doi.org/10.1162/jocn.2010.21595
Wardak, M., Wong, K. P., Shao, W., Dahlbom, M., Kepe, V., Satyamurthy, N., … Huang, S. C. (2010). Movement correction method for human brain PET images: application to quantitative analysis of dynamic 18F-FDDNP scans. Journal of Nuclear Medicine, 51(2), 210–218.https://doi.org/10.2967/jnumed.109.063701
Wihlbäck, A. C., Sundstrom Poromaa, I., Bixo, M., Allard, P., Mjorndal, T., & Spigset, O. (2004). Influence of menstrual cycle on platelet serotonin uptake site and serotonin2A receptor binding. Psychoneuroendocrinology, 29(6), 757–766. https://doi.org/10.1016/S0306-4530(03)00120-3
Wilson, D., da Silva Lobo, D. S., Tavares, H., Gentil, V., & Vallada, H. (2013). Family-based association analysis of serotonin genes in pathological gambling disorder: Evidence of vulnerability risk in the 5HT-2A receptor gene. Journal of Molecular Neuroscience, 49(3), 550–553. https://doi.org/10.1007/s12031-012-9846-x
Wrase, J., Kahnt, T., Schlagenhauf, F., Beck, A., Cohen, M. X., Knutson, B., & Heinz, A. (2007). Different neural systems adjust motor behavior in response to reward and punishment. NeuroImage, 36(4), 1253–1262. https://doi.org/10.1016/j.neuroimage.2007.04.001
Yan, C. G., Cheung, B., Kelly, C., Colcombe, S., Craddock, R. C., Di Martino, A., Li, Q., Nian Zuo, X. N., Castellanos, F. X., & Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. https://doi.org/10.1016/j.neuroimage.2013.03.004
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. https://doi.org/10.1037/0033-295X.111.4.931
Zhao, X., Sun, L., Sun, Y. H., Ren, C., Chen, J., Wu, Z. Q., … Lv, X. L. (2014). Association of HTR2A T102C and A-1438G polymorphisms with susceptibility to major depressive disorder: a meta-analysis. Neurological Sciences, 35(12), 1857–1866.https://doi.org/10.1007/s10072-014-1970-7
Acknowledgements
We thank K. Suzuki, S. Kawakami, and I. Kaneko for assistance as clinical research coordinators, H. Sano for assistance as an MRI technician, and members of the Clinical Imaging Team for support with PET scans. This work was supported in part by the Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency; by the Naito Foundation; by AMED under Grant Number dm0207007 and dm0107094; and by JSPS KAKENHI Grant Number 17H02173.
Funding
This work was supported in part by the Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency; by the Naito Foundation; by AMED under Grant Number dm0207007 and dm0107094; and by JSPS KAKENHI Grant Number 17H02173 and 20H05711; and by the MEXT Quantum Leap Flagship Program (MEXT Q-LEAP) [Grant Number JPMXS0120330644].
Author information
Authors and Affiliations
Contributions
M.Y., Y.K, T.S designed the study; M.Y., Y.K., K.T., T.I., K.Y., H.H., K.Kawamura, M.Z. and H.I. conducted the experiment; K.Kojima., M.Y., Y.K., C.S. and Y.I. analyzed data; K.Kojima, M.Y., S.H., Y.K., S.K., M.H. and T.S. wrote the paper.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All participants provided written informed consent before participating in the study.
Consent to publish
All authors provided their consent to publish this manuscript.
Conflict of interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kojima, K., Hirano, S., Kimura, Y. et al. Brain 5-HT2A receptor binding and its neural network related to behavioral inhibition system. Brain Imaging and Behavior 16, 1337–1348 (2022). https://doi.org/10.1007/s11682-021-00609-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00609-2