Abstract
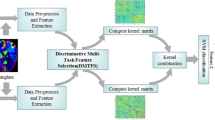
Multimodal classification methods using different modalities of imaging and non-imaging data have recently shown great advantages over traditional single-modality-based ones for diagnosis and prognosis of Alzheimer’s disease (AD), as well as its prodromal stage, i.e., mild cognitive impairment (MCI). However, to the best of our knowledge, most existing methods focus on mining the relationship across multiple modalities of the same subjects, while ignoring the potentially useful relationship across different subjects. Accordingly, in this paper, we propose a novel learning method for multimodal classification of AD/MCI, by fully exploring the relationships across both modalities and subjects. Specifically, our proposed method includes two subsequent components, i.e., label-aligned multi-task feature selection and multimodal classification. In the first step, the feature selection learning from multiple modalities are treated as different learning tasks and a group sparsity regularizer is imposed to jointly select a subset of relevant features. Furthermore, to utilize the discriminative information among labeled subjects, a new label-aligned regularization term is added into the objective function of standard multi-task feature selection, where label-alignment means that all multi-modality subjects with the same class labels should be closer in the new feature-reduced space. In the second step, a multi-kernel support vector machine (SVM) is adopted to fuse the selected features from multi-modality data for final classification. To validate our method, we perform experiments on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database using baseline MRI and FDG-PET imaging data. The experimental results demonstrate that our proposed method achieves better classification performance compared with several state-of-the-art methods for multimodal classification of AD/MCI.







Similar content being viewed by others
References
Al, N. F. E. (2008). Principal component analysis of FDG PET in amnestic MCI. European Journal of Nuclear Medicine and Molecular Imaging, 35(12), 2191–2202 (2112).
Apostolova, L. G., Hwang, K. S., Andrawis, J. P., Green, A. E., Babakchanian, S., Morra, J. H., et al. (2010). 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiology of Aging, 31(8), 1284–1303.
Bouwman, F. H., van der Flier, W. M., Schoonenboom, N. S. M., van Elk, E. J., Kok, A., Rijmen, F., et al. (2007). Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology, 69(10), 1006–1011.
Brookmeyer, R., Johnson, E., Ziegler-Grahamm, K., Arrighi, H. M., Brookmeyer, R., & Johnson, E. (2007). O1-02-01 forecasting the global burden of Alzheimer’s disease. Alzheimers & Dementia the Journal of the Alzheimers Association, 3(3), 186–191.
Chang, C. C., & Lin, C. J. (2007). LIBSVM: a library for support vector machines. ACM Transactions on Intelligent Systems and Technology, 2(3), 389–396.
Chen, X., Pan, W., Kwok, J. T., & Carbonell, J. G. (2009). Accelerated gradient method for multi-task sparse learning problem. Proceedings of the International Conference on Data Mining, 746–751.
Chételat, G., Desgranges, B., Sayette, V., La, D., Viader, F., Eustache, F., & J-C, B. (2003). Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology, 60(8), 1374–1377.
Dai, Z., Yan, C., Wang, Z., Wang, J., Xia, M., Li, K., et al. (2012). Discriminative analysis of early Alzheimer’s disease using multi-modal imaging and multi-level characterization with multi-classifier (M3). NeuroImage, 59(3), 2187–2195.
De, S. S., de Leon, M. J., Rusinek, H., Convit, A., Tarshish, C. Y., Roche, A., et al. (2001). Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiology of Aging, 22(4), 529–539.
Derflinger, S., Sorg, C., Gaser, C., Myers, N., Arsic, M., Kurz, A., et al. (2011). Grey-matter atrophy in Alzheimer’s disease is asymmetric but not lateralized. Journal of Alzheimers Disease, 25(2), 347–357.
Desikan, R. S., Cabral, H. J., Hess, C. P., Dillon, W. P., Glastonbury, C. M., Weiner, M. W., et al. (2009). Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer’s disease. Brain, 132(Part 8), 2048–2057.
Du, A. T., Schuff, N., Kramer, J. H., Rosen, H. J., Gorno-Tempini, M. L., Rankin, K., et al. (2007). Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain, 130(4), 1159–1166.
Evgeniou, T., & Pontil, M. (2004). Regularized multi—task learning. In Proceedings of the tenth ACM SIGKDD international conference on Knowledge discovery and data mining, (pp. 109–117).
Fan, Y., Shen, D., Gur, R. C., Gur, R. E., & Davatzikos, C. (2007). COMPARE: classification of morphological patterns using adaptive regional elements. IEEE Transactions on Medical Imaging, 26(1), 93–105.
Fjell, A. M., Walhovd, K. N. C., Mcevoy, L. K., Hagler, D. J., Holland, D., Brewer, J. B., et al. (2010). CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(6), 2088–2101.
Foster, N. L., Heidebrink, J. L., Clark, C. M., Jagust, W. J., Arnold, S. E., Barbas, N. R., et al. (2007). FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain, 130(10), 2616–2635 (2620).
Gerardin, E., Chételat, G. l., Chupin, M., Cuingnet, R., Desgranges, B., Kim, H. S., et al. (2009). Multidimensional classification of hippocampal shape features discriminates Alzheimer’s disease and mild cognitive impairment from normal aging. NeuroImage, 47(4), 1476–1486.
Gray, K. R., Aljabar, P., Heckemann, R. A., Hammers, A., & Rueckert, D. (2012). Random forest-based similarity measures for multi-modal classification of Alzheimer’s disease. NeuroImage, 65, 167–175.
Higdon, R., Foster, N. L., Koeppe, R. A., DeCarli, C. S., Jagust, W. J., Clark, C. M., et al. (2004). A comparison of classification methods for differentiating fronto-temporal dementia from Alzheimer’s disease using FDG-PET imaging. Statistics in Medicine, 23(2), 315–326. doi:10.1002/sim.1719.
Hinrichs, C., Singh, V., Xu, G., & Johnson, S. C. (2011). Predictive markers for AD in a multi-modality framework: an analysis of MCI progression in the ADNI population. NeuroImage, 55(2), 574–589.
Huang, S., Li, J., Ye, J., Wu, T., Chen, K., & Fleisher, A., et al. (2011). Identifying Alzheimer s disease-related brain regions from multi-modality neuroimaging data using sparse composite linear discrimination analysis. In J. Shawe-Taylor, R. S. Zemel, P. L. Bartlett, F. Pereira, & K. Q. Weinberger (Eds.), Advances in neural information processing systems 24. Curran Associates, Inc.
Jack, C. R., Jr., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9(1), 119–128.
Jie, B., Zhang, D., Cheng, B., & Shen, D. (2015). Manifold regularized multitask feature learning for multimodality disease classification. Human Brain Mapping, 36(2), 489–507.
Kumar, A., & Daume Iii, H. (2012). Learning task grouping and overlap in multi-task learning. Computer Science - Learning.
Landau, S. M., Harvey DMadison, C. M., Reiman, E. M., Foster, N. L., Aisen, P. S., Petersen, R. C., et al. (2010). Comparing predictors of conversion and decline in mild cognitive impairment. Neurology, 75(3), 230–238.
Leon, M. J. D., Mosconi, L., Li, J., Santi, S. D., Yao, Y., Tsui, W. H., et al. (2007). Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. Journal of Neurology, 254(12), 1666–1675.
Liu, J., & Ye, J. (2010). Efficient L1/Lq norm regularization. Cambridge University Pub.
Liu, F., Wee, C. Y., Chen, H., & Shen, D. (2014). Inter-modality relationship constrained multi-modality multi-task feature selection for Alzheimer’s disease and mild cognitive impairment identification. NeuroImage, 84, 466–475.
Magnin, B. t., Mesrob, L., Kinkingnéhun, S., Pélégrini-Issac, M., Colliot, O., Sarazin, M., et al. (2009). Support vector machine-based classification of Alzheimer’s disease from whole-brain anatomical MRI. Neuroradiology, 51(2), 73–83.
Mattsson, N., Zetterberg, H., Hansson, O., Andreasen, N., Parnetti, L., Jonsson, M., et al. (2009). CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA: The Journal of the American Medical Association, 302(4), 385–393.
Mcevoy, L. K., Fennema-Notestine, C., Roddey, J. C., Hagler, D. J., Jr., Holland, D., Karow, D. S., et al. (2009). Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment1. Radiology, 251(1), 195–205.
MJ, W., Kawas, C. H., Stewart, W. F., Rudow, G. L., & Troncoso, J. C. (2004). Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiology of Aging, 25(25), 1205–1212.
Morris, J., Storandt, M., Miller, J., McKeel, D., Price, J., Rubin, E., et al. (2001). Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology, 58(3), 397–405.
Nesterov, Y. (2003). Introductory lectures on convex optimization: a basic course. Computer Programming(Oct), 49–50.
Nestor, P. J., Scheltens, P., & Hodges, J. R. (2004). Advances in the early detection of Alzheimer’s disease. Nature Medicine, 10 suppl(7suppl), S34–S41.
Obozinski, G., Jordan, M., & Taskar, B. (2006). Multi-task feature selection. The Workshop of Structural Knowledge Transfer for Machine Learning in International Conference on Machine Learning, 7(2), 1693–1696.
Obozinski, G., Taskar, B., & Jordan, M. I. (2010). Joint covariate selection and joint subspace selection for multiple classification problems. Statistics and Computing, 20(2), 231–252.
Oliveira, P. P. D., Nitrini, R., Busatto, G., Buchpiguel, C., Sato, J. R., & Amaro, E. (2010). Use of SVM methods with surface-based cortical and volumetric subcortical measurements to detect Alzheimer’s disease. Journal of Alzheimers Disease, 19(4), 1263–1272. doi:10.3233/jad-2010-1322.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology, 56(3), 303–308.
Poulina, S., Dautoffb, R., Morris, J., Barrett, L., & Dickersona, B. (2011). Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Research: Neuroimaging, 194(1), 7–13.
Shattuck, D. W., Sandor-Leahy, S. R., Schaper, K. A., Rottenberg, D. A., & Leahy, R. M. (2001). Magnetic resonance image tissue classification using a partial volume model. In Neuroimage, pp. 856–876.
Shaw, L. M., Vanderstichele, H., Knapik‐Czajka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., et al. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of Neurology, 65(4), 403–413.
Shen, D., & Davatzikos, C. (2002). HAMMER: hierarchical attribute matching mechanism for elastic registration. In IEEE Trans. on Medical Imaging pp. 1421–1439.
Sled, J. G., Zijdenbos, A. P., & Evans, A. C. (1997). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87–97.
Smith, & Stephen, M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155.
Sole, A. D., Clerici, F., Chiti, A., Lecchi, M., Mariani, C., Maggiore, L., et al. (2008). Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. European Journal of Nuclear Medicine and Molecular Imaging, 35(7), 1357–1366.
Suk, H. I., Lee, S. W., & Shen, D. (2014). Subclass-based multi-task learning for Alzheimer’s disease diagnosis. Frontiers in Aging Neuroscience, 6(6), 168.
Tibshirani, R. (1994). Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society, 58(1), 267–288.
Walhovd, K. B., Fjell, A. M., Dale, A. M., Mcevoy, L. K., Brewer, J., Karow, D. S., et al. (2010). Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiology of Aging, 31(7), 1107–1121.
Westman, E., Muehlboeck, J. S., & Simmons, A. (2012). Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. NeuroImage, 62(1), 229–238.
Wolf, H., Jelic, V., Gertz, H. J., Nordberg, A., Julin, P., & Wahlund, L. O. (2003). A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurologica Scandinavica, 179(Supplement s179), 52–76.
Yuan, M., & Lin, Y. (2006). Model selection and estimation in regression with grouped variables. Journal of the Royal Statistical Society, 68(1), 49–67. As the access to this document is restricted, you may want to look for a different version under “Related research” (further below) orfor a different version of it.
Yuan, L., Wang, Y., Thompson, P. M., Narayan, V. A., & Ye, J. (2012). Multi-source feature learning for joint analysis of incomplete multiple heterogeneous neuroimaging data ☆. NeuroImage, 61(3), 622–632.
Zhang, D., & Shen, D. (2012). Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer’s disease. NeuroImage, 59(2), 895–907.
Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57.
Zhang, D., Wang, Y., Zhou, L., Yuan, H., & Shen, D. (2011). Multimodal classification of Alzheimer’s disease and mild cognitive impairment. NeuroImage, 55, 856–867.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (Nos. 61422204, 61473149, 61170151), the Jiangsu Natural Science Foundation for Distinguished Young Scholar (No. BK20130034), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123218110009), and the NUAA Fundamental Research Funds (No. NE2013105), and by NIH grants EB006733, EB008374, EB009634, MH100217, AG041721, and AG042599.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zu, C., Jie, B., Liu, M. et al. Label-aligned multi-task feature learning for multimodal classification of Alzheimer’s disease and mild cognitive impairment. Brain Imaging and Behavior 10, 1148–1159 (2016). https://doi.org/10.1007/s11682-015-9480-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9480-7