Abstract
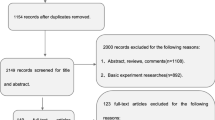
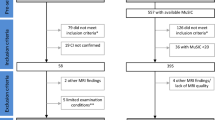
The clinical relevance of gray/white matter contrast ratio (GWR) in mild cognitive impairment (MCI) remains unknown. This study examined baseline GWR and 3-year follow-up diagnostic status in MCI. Alzheimer’s Disease Neuroimaging Initiative MCI participants with baseline 1.5 T MRI and 3-year follow-up clinical data were included. Participants were categorized into two groups based on 3-year follow-up diagnoses: 1) non-converters (n = 69, 75 ± 7, 26 % female), and 2) converters (i.e., dementia at follow-up; n = 69, 75 ± 7, 30 % female) who were matched on baseline age and Mini-Mental State Examination scores. Groups were compared on FreeSurfer generated baseline GWR from structural images in which higher values represent greater tissue contrast. A general linear model, adjusting for APOE-status, scanner type, hippocampal volume, and cortical thickness, revealed that converters evidenced lower GWR values than non-converters (i.e., more degradation in tissue contrast; p = 0.03). Individuals with MCI who convert to dementia have lower baseline GWR values than individuals who remain diagnostically stable over a 3-year period, statistically independent of cortical thickness or hippocampal volume.


Similar content being viewed by others
References
Albert, M. S., Dekosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7, 270–279.
Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R., & Van Hoesen, G. W. (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex in patients with Alzheimer’s disease. Cerebral Cortex, 1, 103–116.
Baron, J. C., Chetelat, G., Desgranges, B., Perchey, G., Landeau, B., de la Sayette, V., et al. (2001). In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. NeuroImage, 14, 298–309.
Beckett, T. L., Webb, R. L., Niedowicz, D. M., Holler, C. J., Matveev, S., Baig, I., et al. (2012). Postmortem Pittsburgh Compound B (PiB) binding increases with Alzheimer’s disease progression. Journal of Alzheimer's Disease, 32, 127–138.
Blackmon, K., Halgren, E., Barr, W. B., Carlson, C., Devinsky, O., DuBois, J., et al. (2011). Individual differences in verbal abilities associated with regional blurring of the left gray and white matter boundary. The Journal of Neuroscience, 31, 15257–15263.
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H., & Del Tredici, K. (2006). Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica, 112, 389–404.
Buckner, R. L., Head, D., Parker, J., Fotenos, A. F., Marcus, D., Morris, J. C., et al. (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23, 724–738.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9, 179–194.
Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97, 11050–11055.
Fischl, B., Sereno, M. I., Tootell, R. B., & Dale, A. M. (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8, 272–284.
Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., et al. (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl 1), S69–84.
Ganguli, M., Snitz, B. E., Saxton, J. A., Chang, C. C., Lee, C. W., Vander Bilt, J., et al. (2011). Outcomes of mild cognitive impairment by definition: a population study. Archives of Neurology, 68, 761–767.
Han, X., Jovicich, J., Salat, D., van der Kouwe, A., Quinn, B., Czanner, S., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32, 180–194.
Herholz, K., Salmon, E., Perani, D., Baron, J. C., Holthoff, V., Frolich, L., et al. (2002). Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. NeuroImage, 17, 302–316.
Hulley, S. B., Cummings, S. R., Browner, W. S., Grady, D. G., & Newman, T. B. (2001). Designing Clinical Research. Philadelphia: Lippincott Williams & Wilkins.
Jack, C.R., Jr., Bernstein, M.A., Fox, N.C., Thompson, P., Alexander, G., Harvey, D., Borowski, B., Britson, P.J., J, L.W., Ward, C., Dale, A.M., Felmlee, J.P., Gunter, J.L., Hill, D.L., Killiany, R., Schuff, N., Fox-Bosetti, S., Lin, C., Studholme, C., DeCarli, C.S., Krueger, G., Ward, H.A., Metzger, G.J., Scott, K.T., Mallozzi, R., Blezek, D., Levy, J., Debbins, J.P., Fleisher, A.S., Albert, M., Green, R., Bartzokis, G., Glover, G., Mugler, J., Weiner, M.W., 2008. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging 27, 685–691.
Jack, C. R., Jr., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9, 119–128.
Killiany, R. J., Moss, M. B., Albert, M. S., Sandor, T., Tieman, J., & Jolesz, F. (1993). Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer’s disease. Archives of Neurology, 50, 949–954.
Kitagaki, H., Mori, E., Hirono, N., Ikejiri, Y., Ishii, K., Imamura, T., et al. (1997). Alteration of white matter MR signal intensity in frontotemporal dementia. American Journal of Neuroradiology, 18, 367–378.
Kong, L., Herold, C., Stieltjes, B., Essig, M., Seidl, U., Wolf, R. C., et al. (2012). Reduced gray to white matter tissue intensity contrast in schizophrenia. PLoS One, 7, e37016.
Lemaitre, H., Goldman, A. L., Sambataro, F., Verchinski, B. A., Meyer-Lindenberg, A., Weinberger, D. R., et al. (2012). Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology Aging, 33(617), e611–619.
Magnaldi, S., Ukmar, M., Vasciaveo, A., Longo, R., & Pozzi-Mucelli, R. S. (1993). Contrast between white and grey matter: MRI appearance with ageing. European Radiology, 3, 513–519.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944.
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology, 74, 201–209.
Raz, N., Millman, D., & Sarpel, G. (1990). Cerebral correlates of cognitive aging: gray-white-matter differentiation in the medial temporal lobes, and fluid versus crystallized abilities. Psychobiology, 18, 475–481.
Risacher, S. L., Saykin, A. J., West, J. D., Shen, L., Firpi, H. A., & McDonald, B. C. (2009). Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research, 6, 347–361.
Rosas, H. D., Liu, A. K., Hersch, S., Glessner, M., Ferrante, R. J., Salat, D. H., et al. (2002). Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology, 58, 695–701.
Rusinek, H., de Leon, M. J., George, A. E., Stylopoulos, L. A., Chandra, R., Smith, G., et al. (1991). Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology, 178, 109–114.
Salat, D. H., Buckner, R. L., Snyder, A. Z., Greve, D. N., Desikan, R. S., Busa, E., et al. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14, 721–730.
Salat, D. H., Greve, D. N., Pacheco, J. L., Quinn, B. T., Helmer, K. G., Buckner, R. L., et al. (2009a). Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage, 44, 1247–1258.
Salat, D. H., Lee, S. Y., van der Kouwe, A. J., Greve, D. N., Fischl, B., & Rosas, H. D. (2009b). Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. NeuroImage, 48, 21–28.
Salat, D. H., Chen, J. J., van der Kouwe, A. J., Greve, D. N., Fischl, B., & Rosas, H. D. (2011). Hippocampal degeneration is associated with temporal and limbic gray matter/white matter tissue contrast in Alzheimer’s disease. NeuroImage, 54, 1795–1802.
Sled, J. G., Zijdenbos, A. P., & Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17, 87–97.
Sperling, R. A., Laviolette, P. S., O’Keefe, K., O’Brien, J., Rentz, D. M., Pihlajamaki, M., et al. (2009). Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron, 63, 178–188.
Thambisetty, M., Wan, J., Carass, A., An, Y., Prince, J. L., & Resnick, S. M. (2010). Longitudinal changes in cortical thickness associated with normal aging. NeuroImage, 52, 1215–1223.
Thesen, T., Quinn, B. T., Carlson, C., Devinsky, O., DuBois, J., McDonald, C. R., et al. (2011). Detection of epileptogenic cortical malformations with surface-based MRI morphometry. PLoS One, 6, e16430.
Thomas, D. C., & Greenland, S. (1983). The relative efficiencies of matched and independent sample designs for case–control studies. Journal of Chronic Diseases, 36, 685–697.
van Norden, A. G., de Laat, K. F., Gons, R. A., van Uden, I. W., van Dijk, E. J., van Oudheusden, L. J., et al. (2011). Causes and consequences of cerebral small vessel disease. The RUN DMC study: A prospective cohort study. Study rationale and protocol. BMC Neurology, 11, 29.
Weiner, M. W., Aisen, P. S., Jack, C. R., Jr., Jagust, W. J., Trojanowski, J. Q., Shaw, L., et al. (2010). The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement, 6(202–211), e207.
Westlye, L. T., Walhovd, K. B., Dale, A. M., Espeseth, T., Reinvang, I., Raz, N., et al. (2009). Increased sensitivity to effects of normal aging and Alzheimer’s disease on cortical thickness by adjustment for local variability in gray/white contrast: a multi-sample MRI study. NeuroImage, 47, 1545–1557.
Wu, L., Rowley, J., Mohades, S., Leuzy, A., Dauar, M. T., Shin, M., et al. (2012). Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One, 7, e47905.
Yip, A. G., McKee, A. C., Green, R. C., Wells, J., Young, H., Cupples, L. A., et al. (2005). APOE, vascular pathology, and the AD brain. Neurology, 65, 259–265.
Acknowledgments
This research was supported by K23-AG030962 (Paul B. Beeson Career Development Award in Aging; ALJ); Alzheimer’s Association IIRG-08-88733 (ALJ); R01-AG034962 (ALJ); R01-HL111516 (ALJ); K24-AG046373 (ALJ); American Federation for Aging Research Medical Student Training in Aging Research Grant (JS; T35-AG038027); R01-NR010827 (DS); and the Vanderbilt Memory & Alzheimer’s Center. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, by the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec, Inc.; Bristol-Myers Squibb Company; Eisai, Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development, LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer, Inc.; Servier; Synarc, Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study Rev October 16, 2012 at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Stephen Damon and Dandan Liu completed statistical analysis.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Rights and permissions
About this article
Cite this article
Jefferson, A.L., Gifford, K.A., Damon, S. et al. Gray & white matter tissue contrast differentiates Mild Cognitive Impairment converters from non-converters. Brain Imaging and Behavior 9, 141–148 (2015). https://doi.org/10.1007/s11682-014-9291-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-014-9291-2