Abstract
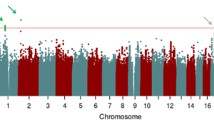
Resilience in executive functioning (EF) is characterized by high EF measured by neuropsychological test performance despite structural brain damage from neurodegenerative conditions. We previously reported single nucleotide polymorphism (SNP) genome-wide association study (GWAS) results for EF resilience. Here, we report gene- and pathway-based analyses of the same resilience phenotype, using an optimal SNP-set (Sequence) Kernel Association Test (SKAT) for gene-based analyses (conservative threshold for genome-wide significance = 0.05/18,123 = 2.8 × 10−6) and the gene-set enrichment package GSA-SNP for biological pathway analyses (False discovery rate (FDR) < 0.05). Gene-based analyses found a genome-wide significant association between RNASE13 and EF resilience (p = 1.33 × 10−7). Genetic pathways involved with dendritic/neuron spine, presynaptic membrane, postsynaptic density, etc., were enriched with association to EF resilience. Although replication of these results is necessary, our findings indicate the potential value of gene- and pathway-based analyses in research on determinants of cognitive resilience.
Similar content being viewed by others
References
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate—a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57(1), 289–300.
Blalock, E. M., Buechel, H. M., Popovic, J., Geddes, J. W., & Landfield, P. W. (2011). Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient Alzheimer’s disease. Journal of Chemical Neuroanatomy, 42(2), 118–126. doi:10.1016/j.jchemneu.2011.06.007.
Crane, P. K., Carle, A., Gibbons, L. E., Insel, P., Mackin, R. S., Gross, A., et al. (2012). Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging and Behavior. doi:10.1007/s11682-012-9186-z.
Debette, S., & Markus, H. S. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ, 341, c3666. doi:10.1136/bmj.c3666.
Elbers, C. C., van Eijk, K. R., Franke, L., Mulder, F., van der Schouw, Y. T., Wijmenga, C., et al. (2009). Using genome-wide pathway analysis to unravel the etiology of complex diseases. Genetic Epidemiology, 33(5), 419–431. doi:10.1002/gepi.20395.
ENIGMA2 Genetics Support Team (2012). NIGMA2 IKGP Cookbook. The Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) consortium.
Fridley, B. L., & Biernacka, J. M. (2011). Gene set analysis of SNP data: benefits, challenges, and future directions. European Journal of Human Genetics, 19(8), 837–843. doi:10.1038/ejhg.2011.57.
Gibbons, L. E., Carle, A. C., Mackin, R. S., Harvey, D., Mukherjee, S., Insel, P., et al. (2012). A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging and Behavior. doi:10.1007/s11682-012-9176-1.
Goeman, J. J., & Bühlmann, P. (2007). Analyzing gene expression data in terms of gene sets: methodological issues. Bioinformatics, 23(8), 980–987. doi:10.1093/bioinformatics/btm051.
Hachinski, V. C., Iliff, L. D., Zilhka, E., Du Boulay, G. H., McAllister, V. L., Marshall, J., et al. (1975). Cerebral blood flow in dementia. Archives of Neurology, 32(9), 632–637.
Harel, A., Wu, F., Mattson, M. P., Morris, C. M., & Yao, P. J. (2008). Evidence for CALM in directing VAMP2 trafficking. Traffic, 9(3), 417–429. doi:10.1111/j.1600-0854.2007.00694.x.
Harold, D., Abraham, R., Hollingworth, P., Sims, R., Gerrish, A., Hamshere, M. L., et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature Genetics, 41(10), 1088–1093. doi:10.1038/ng.440.
Holmans, P. (2010). Statistical methods for pathway analysis of genome-wide data for association with complex genetic traits. Advances in Genetics, 72, 141–179.
Ikram, M. A., Vrooman, H. A., Vernooij, M. W., van der Lijn, F., Hofman, A., van der Lugt, A., et al. (2008). Brain tissue volumes in the general elderly population The Rotterdam Scan Study. Neurobiol Aging, 29(6), 882–890.
Jack, C. R., Jr., Bernstein, M. A., Fox, N. C., Thompson, P., Alexander, G., Harvey, D., et al. (2008). The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging, 27(4), 685–691.
Kim, S. Y., & Volsky, D. J. (2005). PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics, 6, 144. doi:10.1186/1471-2105-6-144.
Knobloch, M., & Mansuy, I. M. (2008). Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Molecular Neurobiology, 37(1), 73–82. doi:10.1007/s12035-008-8018-z.
Kukull, W. A., Higdon, R., Bowen, J. D., McCormick, W. C., Teri, L., Schellenberg, G. D., et al. (2002). Dementia and Alzheimer disease incidence: a prospective cohort study. Archives of Neurology, 59(11), 1737–1746.
Lacor, P. N., Buniel, M. C., Furlow, P. W., Clemente, A. S., Velasco, P. T., Wood, M., et al. (2007). Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(4), 796–807. doi:10.1523/JNEUROSCI.3501-06.2007.
Lee, S., Wu, M. C., & Lin, X. (2012). Optimal tests for rare variant effects in sequencing association studies. Biostatistics, 13(4), 762–775. doi:10.1093/biostatistics/kxs014.
Li, Y., Willer, C. J., Ding, J., Scheet, P., & Abecasis, G. R. (2010). MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic Epidemiology, 34(8), 816–834. doi:10.1002/gepi.20533.
Longstreth, W. T., Jr., Manolio, T. A., Arnold, A., Burke, G. L., Bryan, N., Jungreis, C. A., et al. (1996). Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke, 27(8), 1274–1282.
Longstreth, W. T., Jr., Bernick, C., Manolio, T. A., Bryan, N., Jungreis, C. A., & Price, T. R. (1998). Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Archives of Neurology, 55(9), 1217–1225.
Longstreth, W. T., Jr., Dulberg, C., Manolio, T. A., Lewis, M. R., Beauchamp, N. J., Jr., O’Leary, D., et al. (2002). Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke, 33(10), 2376–2382.
Mukherjee, S., Kim, S., Gibbons, L. E., Nho, K., Risacher, S. L., Glymour, M. M., et al. (2012). Genetic architecture of resilience of executive functioning. Brain Imaging and Behavior. doi:10.1007/s11682-012-9184-1.
Muthén, L., & Muthén, B. (2006). Mplus users guide (41st ed.). Los Angeles: Muthen and Muthen.
Nam, D., Kim, J., Kim, S. Y., & Kim, S. (2010). GSA-SNP: a general approach for gene set analysis of polymorphisms. Nucleic Acids Research, 38(Web Server issue), W749–W754. doi:10.1093/nar/gkq428.
National Bioethics Advisory Commission (2007). http://www.georgetown.edu/research/nrcbl/nbac/. Accessed 7 May 2007.
Negash, S., Bennett, D. A., Wilson, R. S., Schneider, J. A., & Arnold, S. E. (2011). Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory And Aging Project. Current Alzheimer Research, 8(4), 336–340.
Peng, G., Luo, L., Siu, H., Zhu, Y., Hu, P., Hong, S., et al. (2010). Gene and pathway-based second-wave analysis of genome-wide association studies. European Journal of Human Genetics, 18(1), 111–117. doi:10.1038/ejhg.2009.115.
Potkin, S. G., Guffanti, G., Lakatos, A., Turner, J. A., Kruggel, F., Fallon, J. H., et al. (2009). Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One, 4(8), e6501. doi:10.1371/journal.pone.0006501.
Price, A. L., Patterson, N. J., Plenge, R. M., Weinblatt, M. E., Shadick, N. A., & Reich, D. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38(8), 904–909. doi:10.1038/ng1847.
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics, 81(3), 559–575.
Ramanan, V. K., Kim, S., Holohan, K., Shen, L., Nho, K., Risacher, S. L., et al. (2012a). Genome-wide pathway analysis of memory impairment in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging and Behavior, 6(4), 634–648. doi:10.1007/s11682-012-9196-x.
Ramanan, V. K., Shen, L., Moore, J. H., & Saykin, A. J. (2012b). Pathway analysis of genomic data: concepts, methods, and prospects for future development. Trends in Genetics, 28(7), 323–332. doi:10.1016/j.tig.2012.03.004.
Reed, B. R., Mungas, D., Farias, S. T., Harvey, D., Beckett, L., Widaman, K., et al. (2010). Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain: A Journal of Neurology, 133(Pt 8), 2196–2209. doi:10.1093/brain/awq154.
Saykin, A. J., Shen, L., Foroud, T. M., Potkin, S. G., Swaminathan, S., Kim, S., et al. (2010). Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement, 6(3), 265–273. doi:10.1016/j.jalz.2010.03.013.
Scarmeas, N., & Stern, Y. (2003). Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology, 25(5), 625–633.
Shimohama, S., Kamiya, S., Taniguchi, T., Akagawa, K., & Kimura, J. (1997). Differential involvement of synaptic vesicle and presynaptic plasma membrane proteins in Alzheimer’s disease. Biochemical and Biophysical Research Communications, 236(2), 239–242. doi:10.1006/bbrc.1997.6940.
Sonnen, J. A., Larson, E. B., Crane, P. K., Haneuse, S., Li, G., Schellenberg, G. D., et al. (2007). Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of Neurology, 62(4), 406–413.
Sonnen, J. A., Santa Cruz, K., Hemmy, L. S., Woltjer, R., Leverenz, J. B., Montine, K. S., et al. (2011). Ecology of the aging human brain. Archives of Neurology, 68(8), 1049–1056. doi:10.1001/archneurol.2011.157.
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of International Neuropsychological Society, 8(3), 448–460.
Stern, Y. (2003). The concept of cognitive reserve: a catalyst for research. Journal of Clinical and Experimental Neuropsychology, 25(5), 589–593.
The International HapMap Project. (2003). Nature, 426(6968), 789–796. doi:10.1038/nature02168.
Vermeer, S. E., Longstreth, W. T., Jr., & Koudstaal, P. J. (2007). Silent brain infarcts: a systematic review. Lancet Neurology, 6(7), 611–619. doi:10.1016/S1474-4422(07)70170-9.
Weiner, M. W., Aisen, P. S., Jack, C. R., Jr., Jagust, W. J., Trojanowski, J. Q., Shaw, L., et al. (2010). The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement, 6(3), 202–211.e207.
Whalley, L. J., Deary, I. J., Appleton, C. L., & Starr, J. M. (2004). Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews, 3(4), 369–382.
Acknowledgment
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. Data management and the specific analyses reported here were supported by NIH grant R01 AG029672 (Paul Crane, PI), R13 AG030995 (Mungas), and P50 AG05136 (Montine), R01 AG032990 and P50 AG016574 (Crane), from the NIA as well as NIA R01 AG19771 (Saykin) and P30 AG10133 (Saykin/Ghetti).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 182 KB)
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Mukherjee, S., Kim, S., Ramanan, V.K. et al. Gene-based GWAS and biological pathway analysis of the resilience of executive functioning. Brain Imaging and Behavior 8, 110–118 (2014). https://doi.org/10.1007/s11682-013-9259-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-013-9259-7