Abstract
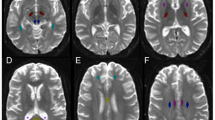
To identify and characterize otherwise occult inter-individual spatial variation of white matter abnormalities across mild traumatic brain injury (mTBI) patients. After informed consent and in compliance with Health Insurance Portability and Accountability Act (HIPAA), Diffusion tensor imaging (DTI) was performed on a 3.0 T MR scanner in 34 mTBI patients (19 women; 19–64 years old) and 30 healthy control subjects. The patients were imaged within 2 weeks of injury, 3 months after injury, and 6 months after injury. Fractional anisotropy (FA) images were analyzed in each patient. To examine white matter diffusion abnormalities across the entire brain of individual patients, we applied Enhanced Z-score Microstructural Assessment for Pathology (EZ-MAP), a voxelwise analysis optimized for the assessment of individual subjects. Our analysis revealed areas of abnormally low or high FA (voxel-wise P-value < 0.05, cluster-wise P-value < 0.01(corrected for multiple comparisons)). The spatial pattern of white matter FA abnormalities varied among patients. Areas of low FA were consistent with known patterns of traumatic axonal injury. Areas of high FA were most frequently detected in the deep and subcortical white matter of the frontal, parietal, and temporal lobes, and in the anterior portions of the corpus callosum. The number of both abnormally low and high FA voxels changed during follow up. Individual subject assessments reveal unique spatial patterns of white matter abnormalities in each patient, attributable to inter-individual differences in anatomy, vulnerability to injury and mechanism of injury. Implications of high FA remain unclear, but may evidence a compensatory mechanism or plasticity in response to injury, rather than a direct manifestation of brain injury.







Similar content being viewed by others
Abbreviations
- CT:
-
Computerized tomography
- DTI:
-
Diffusion tensor imaging
- EZ:
-
Enhanced Z-score
- EZ-MAP:
-
Enhanced Z-score microstructural assessment for pathology
- FA:
-
Fractional anisotropy
- GCS:
-
Glasgow Coma Scale
- GRF:
-
Gaussian Random Field
- HIPAA:
-
Health Insurance Portability and Accountability Act
- IRB:
-
Institutional Review Board
- JHU:
-
Johns Hopkins University
- MNI:
-
Montreal Neurological Institute
- MR:
-
Magnetic resonance
- mTBI:
-
Mild traumatic brain injury
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- TAI:
-
Traumatic axonal injury
- TBI:
-
Traumatic brain injury
References
Ardekani, B. (1995). A fully automatic multimodality image registration algorithm. Journal of Computer Assisted Tomography, 19(4), 615–623.
Ardekani, B., Guckemus, S., Bachman, A., Hoptman, M. J., Wojtaszek, M., & Nierenberg, J. (2005). Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. Journal of Neuroscience Methods, 142(1), 67–76.
Bazarian, J. J., Zhong, J., Blyth, B., Zhu, T., Kavcic, V., & Peterson, D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. [Article]. Journal of Neurotrauma, 24(9), 1447–1459. doi:10.1089/neu.2007.0241.
Bennett, R. E., Mac Donald, C. L., & Brody, D. L. (2012). Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neuroscience Letters. doi:10.1016/j.neulet.2012.02.024.
Bigler, E. D. & Maxwell, W. L. (2012). Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging and Behavior. This special issue.
Budde, M. D., Janes, L., Gold, E., Turtzo, L. C., & Frank, J. A. (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t]. Brain: A journal of neurology, 134(Pt 8), 2248–2260. doi:10.1093/brain/awr161.
Crooks, D. (1991). The pathological concept of diffuse axonal injury: its pathogenesis and the assessment of severity. The Journal of Pathology, 165(1), 5–10.
Esselman, P., & Uomoto, J. M. (1995). Classification of the spectrum of mild traumatic brain injury. Brain Injury, 9(4), 417–424.
Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C., & Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1, 210–220.
Geary, E. K., Kraus, M. F., Pliskin, N. H., & Little, D. M. (2010). Verbal learning differences in chronic mild traumatic brain injury. Journal of the International Neuropsychological Society, 16(3), 506–516. doi:10.1017/S135561771000010X.
Greer, J. E., McGinn, M. J., & Povlishock, J. T. (2011). Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. Journal of Neuroscience, 31(13), 5089–5105. doi:10.1523/jneurosci.5103-10.2011.
Hammoud, D., & Wasserman, B. A. (2002). Diffuse axonal injuries: a pathophysiology and imaging. Neuroimaging Clinics of North America, 12(2), 205–216.
Hartikainen, K. M., Waljas, M., Isoviita, T., Dastidar, P., Liimatainen, S., Solbakk, A. K., et al. (2010). Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. Journal of Clinical and Experimental Neuropsychology, 1–8. doi:10.1080/13803390903521000.
Holmes, C., Hoge, R., Collins, L., Woods, R., Toga, A. W., & Evans, A. C. (1998). Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography, 22(2), 324–333.
Inglese, M., Makani, S., Johnson, G., Cohen, B. A., Silver, J. A., Gonen, O., et al. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of Neurosurgery, 103(2), 298–303.
Kim, N., Hulkower, M. B., Park, Y., Gardin, T. M., Smith, J. L., Branch, C. A., et al. (2011). Robust Detection of White Matter Injury in Individual Patients After Mild Traumatic Brain Injury Paper presented at the ISMRM 19th Annual Meeting and Exhibition, Montreal, Canada, May 9, 2011.
Kou, Z., Wu, Z., Tong, K. A., Holshouser, B., Benson, R. R., Hu, J., et al. (2010). The role of advanced MR imaging findings as biomarkers of traumatic brain injury. The Journal of Head Trauma Rehabilitation, 25(4), 267–282. doi:10.1097/HTR.0b013e3181e54793.
Kraus, M. F., Susmaras, T., Caughlin, B. P., Walker, C. J., Sweeney, J. A., & Little, D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. [Article]. Brain, 130, 2508–2519. doi:10.1093/brain/awm216.
Levin, H. S., Wilde, E., Troyanskaya, M., Petersen, N. J., Scheibel, R., Newsome, M., et al. (2010). Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma, 27(4), 683–694. doi:doi:10.1089/neu.2009.1073.
Lim, K., Ardekani, B. A., Nierenberg, J., Butler, P. D., Javitt, D. C., & Hoptman, M. J. (2006). Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. The American Journal of Psychiatry, 163(11), 2008–2010.
Lipton, M. L., Gulko, E., Zimmerman, M. E., Friedman, B. W., Kim, M., Gelella, E., et al. (2009). Diffusion tensor imaging implicates prefrontal axonal injury in executive function impairment following mild traumatic brain injury. Radiology, 252(3), 816–824.
Little, D. M., Kraus, M. F., Joseph, J., Geary, E. K., Susmaras, T., Zhou, X. J., et al. (2010). Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology, 74(7), 558–564. doi:10.1212/WNL.0b013e3181cff5d5.
Lo, C., Shifteh, K., Gold, T., Bello, J. A., & Lipton, M. L. (2009). Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. Journal of Computer Assisted Tomography, 33(2), 293–297.
Mac Donald, C., Dikranian, K., Bayly, P., Holtzman, D., & Brody, D. (2007a). Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. Journal of Neuroscience, 27(44), 11869–11876.
Mac Donald, C., Dikranian, K., Song, S. K., Bayly, P. V., Holtzman, D. M., & Brody, D. L. (2007b). Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Experimental Neurology, 205(1), 116–131.
Mac Donald, C. L., Dikranian, K., Bayly, P., Holtzman, D., & Brody, D. (2007c). Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. Journal of Neuroscience, 27(44), 11869–11876. doi:10.1523/jneurosci.3647-07.2007.
Mayer, A. R., Ling, J., Mannell, M. V., Gasparovic, C., Phillips, J. P., Doezema, D., et al. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology, 74(8), 643–650. doi:10.1212/WNL.0b013e3181d0ccdd.
McArthur, D., Chute, D. J., & Villablanca, J. P. (2004). Moderate and severe traumatic brain injury: epidemiologic, imaging and neuropathologic perspectives. Brain Pathology, 14(2), 185–194.
Meythaler, J. M., Peduzzi, J. D., Eleftheriou, E., & Novack, T. A. (2001). Current concepts: diffuse axonal injury-associated traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 82(10), 1461–1471.
Miles, L., Grossman, R. I., Johnson, G., Babb, J. S., Diller, L., & Inglese, M. (2008). Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Injury, 22(2), 115–122.
Muller, H. P., Unrath, A., Riecker, A., Pinkhardt, E. H., Ludolph, A. C., & Kassubek, J. (2009). Intersubject variability in the analysis of diffusion tensor images at the group level: fractional anisotropy mapping and fiber tracking techniques. Magnetic Resonance Imaging, 27(3), 324–334. doi:10.1016/j.mri.2008.07.003.
Niogi, S. N., Mukherjee, P., Ghajar, J., Johnson, C., Kolster, R. A., Sarkar, R., et al. (2008a). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR. American Journal of Neuroradiology, 29(5), 967–973. doi:10.3174/ajnr.A0970.
Niogi, S. N., Mukherjee, P., Ghajar, J., Johnson, C. E., Kolster, R., Lee, H., et al. (2008b). Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain, 131(Pt 12), 3209–3221. doi:10.1093/brain/awn247.
Oishi, K., Faria, A. V., & Mori, S. (2010). JHU-MNI-ss Atlas.
Pettus, E., Christman, C. W., Giebel, M. L., & Povlishock, J. T. (1994). Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. Journal of Neurotrauma, 11(5), 507–522.
Poupon, C., Clark, C. A., Frouin, V., Regis, J., Bloch, I., Le Bihan, D., et al. (2000). Regularization of diffusion-based direction maps for the tracking of brain white matter fascicles. NeuroImage, 12(2), 184–195. doi:10.1006/nimg.2000.0607.
Povlishock, J. (1986). Traumatically induced axonal damage without concomitant change in focally related neuronal somata and dendrites. Acta Neuropathologica, 70(1), 53–59.
Povlishock, J. (1992). Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathology, 2(1), 1–12.
Povlishock, J., & Katz, D. I. (2005). Update of neuropathology and neurological recovery after traumatic brain injury. The Journal of Head Trauma Rehabilitation, 20(1), 76–94.
Povlishock, J. T., Becker, D. P., Cheng, C. L., & Vaughan, G. W. (1983). Axonal change in minor head injury. Journal of Neuropathology and Experimental Neurology, 42(3), 225–242.
Rosenbaum, S. B. & Lipton, M. L. (2012). Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging and Behavior. This special issue.
Rubovitch, V., Ten-Bosch, M., Zohar, O., Harrison, C. R., Tempel-Brami, C., Stein, E., et al. (2011). A mouse model of blast-induced mild traumatic brain injury. Experimental Neurology, 232(2), 280–289. doi:10.1016/j.expneurol.2011.09.018.
Rutgers, D. R., Toulgoat, F., Cazejust, J., Fillard, P., Lasjaunias, P., & Ducreux, D. (2008). White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. [Proceedings Paper]. American Journal of Neuroradiology, 29(3), 514–519. doi:10.3174/ajnr.A0856.
Scholz, J., Klein, M. C., Behrens, T. E., & Johansen-Berg, H. (2009). Training induces changes in white-matter architecture. [Research Support, Non-U.S. Gov’t]. Nature Neuroscience, 12(11), 1370–1371. 10.1038/nn.2412.
Sharp, D. J., & Ham, T. E. (2011). Investigating white matter injury after mild traumatic brain injury. Current Opinion in Neurology, 24(6), 558–563. doi:10.1097/WCO.0b013e32834cd523.
Shenton, M. E., Hamoda, H. M., Schneiderman, J. S., Bouix, S., Pasternak, O., Rathi, Y., et al. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. This special issue.
Smith, S., Jenkinson, M., Woolrich, M. W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(suppl 1), S208–S219.
Smith, S., Johansen-Berg, H., Jenkinson, M., et al. (2007). Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols, 2(3), 499–503.
Song, S., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20(3), 1714–1722.
Spain, A., Daumas, S., Lifshitz, J., Rhodes, J., Andrews, P. J., Horsburgh, K., et al. (2010). Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. Journal of Neurotrauma, 27(8), 1429–1438. doi:10.1089/neu.2010.1288.
Wang, S., Wu, E. X., Qiu, D., Leung, L. H., Lau, H. F., & Khong, P. L. (2009). Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Research, 69(3), 1190–1198.
Author information
Authors and Affiliations
Corresponding author
Appendix: Data analysis procedures
Appendix: Data analysis procedures
Adjustment for demographic covariate effects
We chose control subjects with an even distribution of age, gender and educational attainment that fully brackets the range of the patients; no patient age or educational attainment exceeds all controls at either extreme. FA images used in subsequent analyses were adjusted by regression coefficients (age, gender, years of education) estimated from control subjects at each voxel. Regression coefficients thus determined were applied to FA images of the patients, but only at locations where effects on individual voxels were significant at p < 0.05 and where more than 100 significant voxels formed a contiguous cluster.
Enhanced Z-score (EZ)
We computed the Z-score defined by \( {Z} = {{{({X} - mean)}} \left/ {{SD}} \right.} \) at each voxel (i) within a patient’s FA volume with reference values (mean and Standard Deviation (SD)) computed from the control group. It therefore follows that Z-score of a patient may vary with the composition of the control group, with potential for unreliable inferences when the reference group is small. We employed a bootstrap procedure to overcome this potential for sample-to-sample variation of Z-scores, and calculated EZ-score by \( E{Z} = {{{{Z}}} \left/ {{\widehat{\sigma}}} \right.} \), where \( \widehat{\sigma} \) is the bootstrap SD estimate of Z-scores at a voxel. We applied two levels of thresholding to identify significantly abnormal voxels. First, each voxel must meet a threshold, |EZ| > 1.96. Second, the subset of these voxels that forms contiguous clusters meeting a size threshold (1 %) based on the Gaussian Random Field (GRF) theory (Friston et al. 1994); the cluster size threshold is corrected for multiple comparisons. These thresholds provide maximal discrimination of patients and controls based on maximal area under the ROC curve (results presented previously (Kim et al. 2011)) determined from a range of thresholds ( voxel z-score: 2.5758 and 1.96; cluster size: 0.01 (corrected), 0.05 (corrected), 0.01 (uncorrected) and 0.05 (uncorrected)). It is important to recognize that some voxels meeting the criteria for abnormality are found in controls when these optimal thresholds are applied. We therefore tested the difference in the numbers of abnormal voxels between patients (n = 34) and unique normal control subjects (n = 21) (i.e., not the same individuals used to compute the reference mean and SD for use in the E-Z calculation). The mean number of abnormal voxels found in patients was significantly greater than that of controls (p = 0.014, 2-tails) (Kim et al. 2011).
One Sample t-Test:
An Enhanced Z-score (EZi) of ith mTBI patient at a voxel (voxel location index is omitted) is written as \( E{Z_i} = \frac{{{X_i} - \overline Y }}{{{S_Y}{{\widehat{\sigma }}^B}}} \), where Xi is FA of ith patient; (\( \overline Y, {S_Y} \)) is mean and SD of control group, respectively; \( {\widehat{\sigma }^B} \)is bootstrap SD estimate of Z-scores. T-score for the 1-sample t-Test with null hypothesis that is the mean Enhanced Z-scores of patients would be equal to zero is written as
since \( SD(E{Z_i}) = \frac{{{S_X}}}{{{S_Y}{{\widehat{\sigma }}^B}}} \) (SD of Enhanced Z-scores of patients), where Sx is SD of FAs from patients, and nx is the number of patient.
Rights and permissions
About this article
Cite this article
Lipton, M.L., Kim, N., Park, Y.K. et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: Intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging and Behavior 6, 329–342 (2012). https://doi.org/10.1007/s11682-012-9175-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9175-2