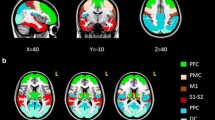
Abstract
The mediodorsal thalamic nucleus is recognized as an association hub mediating interconnections with mainly the prefrontal cortex. Tracer studies in primates and in vivo diffusion tensor tractography findings in both humans and monkeys confirm its role in relaying networks that connect to the dorsolateral prefrontal, orbitofrontal, frontal medial and cingulate cortex. Our study was designed to use in vivo probabilistic tractography to describe the pathways emerging from or projecting to the mediodorsal nucleus; moreover, to use such information to automatically define subdivisions based on the divergence of remote structural connections. Diffusion tensor MR imaging data of 156 subjects were utilized to perform connectivity-based segmentation of the mediodorsal nucleus by employing a k-means clustering algorithm. Two domains were revealed (medial and lateral) that are separated from each other by a sagittally oriented plane. For each subject, general assessment of cognitive performance by means of the Wechsler Abbreviated Scale of Intelligence and measures of Delis-Kaplan Executive Function System (D-KEFS) test was utilized. Inter-subject variability in terms of connectivity-based cluster sizes was discovered and the relative sizes of the lateral mediodorsal domain correlated with the individuals’ performance in the D-KEFS Sorting test (r = 0.232, p = 0.004). Our results show that the connectivity-based parcellation technique applied to the mediodorsal thalamic nucleus delivers a single subject level descriptor of connectional topography; furthermore, we revealed a possible weak interaction between executive performance and the size of the thalamic area from which pathways converge to the lateral prefrontal cortex.



Similar content being viewed by others
References
Aggleton, J. P., & Mishkin, M. (1984). Projections of the amygdala to the thalamus in the cynomolgus monkey. The Journal of Comparative Neurology, 222(1), 56–68.
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9(1), 357–381.
Barbas, H., Henion, T. H. H., & Dermon, C. R. (1991). Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology, 313(1), 65–94.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219.
Behrens, T. E. J., Johansen-Berg, H., Woolrich, M. W., Smith, S. M., Wheeler-Kingshott, C., Boulby, P. A., & Matthews, P. M. (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6(7), 750–757.
Behrens, T. E. J., Woolrich, M. W., Jenkinson, M., Johansen-Berg, H., Nunes, R. G., Clare, S., & Smith, S. M. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088.
Castellanos, F. X., Leventhal, B., & Milham, M. (2011). Nathan Kline Institute (NKI)/Rockland Sample. http://fcon_1000.projects.nitrc.org/indi/pro/nki.html Accessed 01 July 2011.
Catani, M. (2007). From hodology to function. Brain, 130(3), 602–605.
Catani, M., Howard, R. J., Pajevic, S., & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage, 17(1), 77–94.
Croxson, P. L., Johansen-Berg, H., Behrens, T. E. J., Robson, M. D., Pinsk, M. A., Gross, C. G., & Rushworth, M. F. S. (2005). Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. The Journal of Neuroscience, 25(39), 8854–8866.
Dauguet, J., Peled, S., Berezovskii, V., Delzescaux, T., Warfield, S. K., Born, R., & Westin, C. (2007). Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. NeuroImage, 37(2), 530–538.
Delis, D. C., Kramer, J. H., Kaplan, E., & Holdnack, J. (2004). Reliability and validity of the DelisKaplan Executive Function System: an update. Journal of the International Neuropsychological Society, 10, 301–303.
Draganski, B., Kherif, F., Kloppel, S., Cook, P. A., Alexander, D. C., Parker, G. J. M., & Frackowiak, R. S. J. (2008). Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience, 28(28), 7143–7152.
Elias, W. J., Zheng, Z. A., Domer, P., Quigg, M., & Pouratian, N. (2011). Validation of connectivity-based thalamic segmentation with direct electrophysiologic recordings from human sensory thalamus. NeuroImage, doi:10.1016/j.neuroimage.2011.10.049
Erickson, S. L., & Lewis, D. A. (2004). Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. The Journal of Comparative Neurology, 473(1), 107–127.
Giguere, M., & Goldman-Rakic, P. S. (1988). Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. The Journal of Comparative Neurology, 277(2), 195–213.
Gold, B. T., Powell, D. K., Xuan, L., Jiang, Y., & Hardy, P. A. (2007). Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia, 45(11), 2439–2446.
Goldman-Rakic, P. S., & Porrino, L. J. (1985). The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. The Journal of Comparative Neurology, 242(4), 535–560.
Gower, E. C. (1989). Efferent projections from limbic cortex of the temporal pole to the magnocellular medial dorsal nucleus in the rhesus monkey. The Journal of Comparative Neurology, 280(3), 343–358.
Hansen, B., Flint, J. J., Heon-Lee, C., Fey, M., Vincent, F., King, M. A., & Blackband, S. J. (2011). Diffusion tensor microscopy in human nervous tissue with quantitative correlation based on direct histological comparison. NeuroImage, 57(4), 1458–1465.
Izquierdo, A., & Murray, E. A. (2010). Functional interaction of medial mediodorsal thalamic nucleus but not nucleus accumbens with amygdala and orbital prefrontal cortex is essential for adaptive response selection after reinforcer devaluation. The Journal of Neuroscience, 30(2), 661–669.
Jakab, A., Molnar, P., Bogner, P., Beres, M., & Berenyi, E. (2011). Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topography, doi:10.1007/s10548-011-0205-y
Jbabdi, S., Woolrich, M. W., & Behrens, T. E. J. (2009). Multiple-subjects connectivity-based parcellation using hierarchical dirichlet process mixture models. NeuroImage, 44(2), 373–384.
Johansen-Berg, H., & Rushworth, M. F. S. (2009). Using diffusion imaging to study human connectional anatomy. Annual Review of Neuroscience, 32(1), 75–94.
Johansen-Berg, H., Behrens, T. E. J., Robson, M. D., Drobnjak, I., Rushworth, M. F. S., Brady, J. M., & Matthews, P. M. (2004). Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(36), 13335–13340.
Johansen-Berg, H., Behrens, T. E. J., Sillery, E., Ciccarelli, O., Thompson, A. J., Smith, S. M., & Matthews, P. M. (2005). Functional–Anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cerebral Cortex, 15(1), 31–39.
Jung, R. E., & Haier, R. J. (2007). The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. The Behavioral and Brain Sciences, 30(2), 135–154.
Kievit, J., & Kuypers, H. (1977). Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Experimental Brain Research, 29(3), 299–322.
Klein, J. C., Behrens, T. E. J., Robson, M. D., Mackay, C. E., Higham, D. J., & Johansen-Berg, H. (2007). Connectivity-based parcellation of human cortex using diffusion MRI: establishing reproducibility, validity and observer independence in BA 44/45 and SMA/pre-SMA. NeuroImage, 34(1), 204–211.
Klein, J. C., Rushworth, M. F. S., Behrens, T. E. J., Mackay, C. E., de Crespigny, A. J., D’Arceuil, H., & Johansen-Berg, H. (2010). Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. NeuroImage, 51(2), 555–564.
Krauth, A., Blanc, R., Poveda, A., Jeanmonod, D., Morel, A., & Székely, G. (2010). A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. NeuroImage, 49(3), 2053–2062.
Le Bihan, D., Breton, E., Lallemand, D., Grenier, P., Cabanis, E., & Laval-Jeantet, M. (1986). MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology, 161(2), 401–407.
Liang, P., Wang, Z., Yang, Y., Jia, X., & Li, K. (2011). Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One, 6(7), e22153.
Little, D. M., Kraus, M. F., Joseph, J., Geary, E. K., Susmaras, T., Zhou, X. J., & Gorelick, P. B. (2010). Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology, 74(7), 558–564.
Luders, E., Narr, K. L., Thompson, P. M., & Toga, A. W. (2009). Neuroanatomical correlates of intelligence. Intelligence, 37(2), 156–163.
Markowitsch, H. J., Irle, E., & Streicher, M. (1982). The thalamic mediodorsal nucleus receives input from thalamic and cortical regions related to vision. Neuroscience Letters, 32(2), 131–136.
Masterman, D. L., & Cummings, J. L. (1997). Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. Journal of Psychopharmacology, 11(2), 107–114.
Menke, R. A., Jbabdi, S., Miller, K. L., Matthews, P. M., & Zarei, M. (2010). Connectivity-based segmentation of the substantia nigra in human and its implications in parkinson’s disease. NeuroImage, 52(4), 1175–1180.
Metzger, C. D., Eckert, U., Steiner, J., Sartorius, A., Buchmann, J. E., Stadler, J., & Walter, M. (2010). High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in Neuroanatomy. doi:10.3389/fnana.2010.00138.
Morel, A. (2007). Stereotactic atlas of the human thalamus and basal Ganglia. New York: Informa Healthcare USA, inc.
Mori, S., & van Zijl, P. C. M. (2002). Fiber tracking: principles and strategies? a technical review. NMR in Biomedicine, 15(7–8), 468–480.
Nanetti, L., Cerliani, L., Gazzola, V., Renken, R., & Keysers, C. (2009). Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage, 47(4), 1666–1677.
Negyessy, L., & Goldman-Rakic, P. (2005). Morphometric characterization of synapses in the primate prefrontal cortex formed by afferents from the mediodorsal thalamic nucleus. Experimental Brain Research, 164(2), 148–154.
Niemann, K., Mennicken, V. R., Jeanmonod, D., & Morel, A. (2000). The morel stereotactic atlas of the human thalamus: atlas-to-MR registration of internally consistent canonical model. NeuroImage, 12(6), 601–616.
O’Muircheartaigh, J., Vollmar, C., Traynor, C., Barker, G. J., Kumari, V., Symms, M. R., & Richardson, M. P. (2011). Clustering probabilistic tractograms using independent component analysis applied to the thalamus. NeuroImage, 54(3), 2020–2032.
Öngür, D., & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10(3), 206–219.
Pouratian, N., Zheng, Z., Bari, A. A., Behnke, E., Elias, W. J., & DeSalles, A. A. F. (2011). Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. Journal of Neurosurgery, 1–10. doi:10.3171/2011.7.JNS11250.
Pulsipher, D. T., Seidenberg, M., Guidotti, L., Tuchscherer, V. N., Morton, J., Sheth, R. D., & Hermann, B. (2009). Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia, 50(5), 1210–1219.
Radanovic, M., Azambuja, M., Mansur, L. L., Porto, C. S., & Scaff, M. (2003). Thalamus and language: interface with attention, memory and executive functions. Arquivos De Neuro-Psiquiatria, 61(1), 34–42.
Rao, A., Aljabar, P., & Rueckert, D. (2008). Hierarchical statistical shape analysis and prediction of sub-cortical brain structures. Medical Image Analysis, 12(1), 55–68.
Ray, J. P., & Price, J. L. (1993). The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 337(1), 1–31.
Siwek, D. F., & Pandya, D. N. (1991). Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. The Journal of Comparative Neurology, 312(4), 509–524.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., Bannister, P. R., De Luca, M., Drobnjak, I., Flitney, D. E., Niazy, R., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J. M., & Matthews, P. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–19.
Tekin, S., & Cummings, J. L. (2002). Frontal–subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research, 53(2), 647–654.
Tomassini, V., Jbabdi, S., Klein, J. C., Behrens, T. E. J., Pozzilli, C., Matthews, P. M., & Johansen-Berg, H. (2007). Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. The Journal of Neuroscience, 27(38), 10259–10269.
Traynor, C., Heckemann, R. A., Hammers, A., O’Muircheartaigh, J., Crum, W. R., Barker, G. J., & Richardson, M. P. (2010). Reproducibility of thalamic segmentation based on probabilistic tractography. NeuroImage, 52(1), 69–85.
Tuch, D. S., Reese, T. G., Wiegell, M. R., Makris, N., Belliveau, J. W., & Wedeen, V. J. (2002). High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine, 48, 577–582.
Turken, A. U., Whitfield-Gabrieli, S., Bammer, R., Baldo, J. V., Dronkers, N. F., & Gabrieli, J. D. E. (2008). Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage, 42(2), 1032–1044.
Van der Werf, Y. D., Witter, M. P., Uylings, H. B. M., & Jolles, J. (2000). Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia, 38(5), 613–627.
Van der Werf, Y. D., Scheltens, P., Lindeboom, J., Witter, M. P., Uylings, H. B. M., & Jolles, J. (2003). Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia, 41(10), 1330–1344.
Wechsler, D. (1999). Wechsler abbreviated scale of intelligence. San Antonio: The Psychological Corporation.
Wedeen, V. J., Wang, R. P., Schmahmann, J. D., Benner, T., Tseng, W. Y., Dai, G., Pandya, D. N., Hagmann, P., D’Arceuil, H., & de Crespigny, A. J. (2008). Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage, 41(4), 1267–1277.
Wunderlich, K., Schneider, K. A., & Kastner, S. (2005). Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nature Neuroscience, 8(11), 1595–1602.
Yeterian, E. H., & Pandya, D. N. (1988). Corticothalamic connections of paralimbic regions in the rhesus monkey. The Journal of Comparative Neurology, 269(1), 130–146.
Ystad, M., Hodneland, E., Adolfsdottir, S., Haász, J., Lundervold, A. J., Eichele, T., & Lundervold, A. (2011). Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. NeuroImage, 55(1), 24–31.
Acknowledgements
The authors gratefully acknowledge the valuable comments of Anne Morel (Center for Clinical Research, University Hospital Zürich) and the technical support of Saad Jbabdi (Centre for Functional Magnetic Imaging of the Brain, University of Oxford) and Gabor Szekely (Computer Vision Laboratory, ETH Zürich). Subject data were kindly provided by the Nathan S. Kline Institute for Psychiatric research. A.J. is supported by the Sciex NMS-CH Fellowship.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Suppl. Fig. 1
Three-dimensional representation of the connectivity-based clusters of the mediodorsal nucleus, 5th percentile, median and 95th percentile volumes of the MDlat (red objects) and MDmed (blue objects). The atlas-based MDmc and MDpc nuclei borders have been transformed to the standard neuroimaging space (green objects). The Dice overlap measure of the MDlat and MDpc volumes were 0.74 ± 0.04 and 0.45 ± 0.11 for the MDmc – MDmed. (JPEG 187 kb)

Suppl. Fig. 2
Three-dimensional representation of the fiber trajectories emerging from the MDlat and MDmed clusters (depicted in red and blue overlay, respectively). Volume rendering technique was used to demonstrate the averaged fiber anatomy as an overlay on the MNI152 T1-weighted brain template. Top row: lateral-oblique view, bottom row: medial (mid-sagittal) view of the brain template and the resulting networks. (JPEG 265 kb)

Suppl. Fig. 3
Connectivity-based clustering of the MD nucleus as a depiction of individual connectional anatomy. We demonstrate the procedure in two individual cases with different D-KEFS Sorting Test scores. On the left side, a case with high performance is exemplified while the right panels show a low performer. Top row: reordered cross-correlation matrix of the connections emerging from the MD and connectivity-based clusters depicted on the subject’s 3DT1 MRI image. Middle and bottom rows: 3D representations of individual cortical anatomy (volume rendering) and fiber tracts originating from the MDlat (red) and MDmed (blue) subdivisions. (JPEG 1605 kb)
Rights and permissions
About this article
Cite this article
Jakab, A., Blanc, R. & Berényi, E.L. Mapping changes of in vivo connectivity patterns in the human mediodorsal thalamus: correlations with higher cognitive and executive functions. Brain Imaging and Behavior 6, 472–483 (2012). https://doi.org/10.1007/s11682-012-9172-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9172-5