Abstract
The breaking of dormancy mediated by reactive nitrogen species (RNS) is related to the accumulation of reactive oxygen species (ROS) in germinating embryos but the underlying mechanism is unclear. The objectives of this study were: (1) to explore the relationship between RNS-mediated dormancy release and ROS accumulation in germinating embryos of Sorbus pohuashanensis; and, (2) to investigate the relationships among germination time, ROS metabolism, and endogenous hormone synthesis. We studied the effects of exogenous nitric oxide (NO) donor sodium nitroprusside (SNP), the NO scavenger (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), abscisic acid (ABA), the exogenous ethylene donor ethrel, and the ethylene receptor inhibitor 2,5-norbornadien (NBD) on embryo germination and seedling growth. Embryos were released from dormancy by pretreatment with NO or ethylene, which was related to increased ethylene biosynthesis and decreased ABA levels. Breaking of dormancy by SNP was related to increased levels of ethylene, hydrogen peroxide, and glutathione, increased activities of superoxide dismutase and glutathione peroxidase, and decreased levels of ABA, superoxide anions, and malondialdehyde. These effects of nitric oxide were especially significant in seedling hypocotyls and radicles. These results demonstrate that NO can break S. pohuashanensis embryo dormancy by inducing ethylene biosynthesis, and that this signalling pathway is closely related to ROS accumulation and the antioxidant defence response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are emerging as important regulators of plant development (germination, flowering, senescence) that function as secondary messenger in cooperation with classical phytohormones (Gniazdowska et al. 2010). Both ROS and RNS play signalling roles in alleviating seed dormancy, thereby promoting germination (Ishibashi et al. 2017). Hydrogen peroxide (H2O2) also plays a positive role as a signalling molecule in the seed germination of several plant species such as Hordeum vulgare L. (Ishibashi et al. 2017), Vigna radiata (L.) R. Wilczek (Chaudhuri et al. 2013), and Oryza sativa L. cv. Hitomebore (Sasaki et al. 2015). The promoting effects of inorganic nitrates and other nitrogen-containing compounds on germination are thought to represent the release of seed dormancy via a process involving nitric oxide (NO) (Beligni and Lamattina 2000; Bethke et al. 2004). Sodium nitroprusside (SNP) is an exogenous NO donor that promotes seed germination in many plant species (Beligni and Lamattina 2000; Bethke et al. 2004; Gniazdowska et al. 2010; Yang et al. 2013). Nitric oxide has a concentration-dependent effect on seed germination, i.e., a low concentration promotes germination whereas a high concentration inhibits it (Giba et al. 2007). Previous studies have focused on the mechanism by which exogenous H2O2 stimulates germination in O. sativa (Sasaki et al. 2015) and H. vulgare (Ishibashi et al. 2017), and early seedling growth in V. radiata (Chaudhuri et al. 2013). However, few studies have focused on the role of NO and associated factors in seed dormancy and germination.
Mountain ash (Sorbus pohuashanensis (Hance) Hedl.), a deciduous species in the genus Sorbus (Maloideae, Rosaceae), is an economically valuable species for ornamental and medicinal purposes, and parts of the tree are edible (Yang and Shen 2011). Naturally growing S. pohuashanensis is mainly distributed in the north to northeast of China and also sporadically in northern Korea and eastern Russia (Yang et al. 2012a, b). It is a companion species that grows in small sporadic populations with a small population density. S. pohuashanensis is sensitive to high-intensity interspecific competition and has a poor update capability, for example, the number of seedlings is rare under natural conditions (Mala et al. 2009; Xu et al. 2010; Yang et al. 2012a, b). Because of its ornamental value, S. pohuashanensis has gradually gained more attention. Exploitation of the wild resource in the eastern part of north-eastern China has caused great damage, and a portion of the population is on the verge of disappearing.
In previous studies, we have analysed the physiological dormancy characteristics of S. pohuashanensis embryos (Yang and Shen 2011). It takes 105 days (seeds sealed and stored at low temperature) to 120 days (newly harvested mature seeds) to germinate after cold stratification at 2–5 °C (Yang et al. 2008a, b). During the process of cold stratification, the ABA level in the seed coat decreases significantly, and the relative levels of auxin, cytokinin, and gibberellin increase significantly in both seed coat and embryo (Yang et al. 2008a, b). In studies on the effects of various pre-treatments on germination, two-thirds (67%) of S. pohuashanensis seeds soaked in 4% KNO3 for 48 h germinated under variable temperatures from 25 to 5 °C (Bian et al. 2013). Pre-treatment of S. pohuashanensis embryos with 5 mmol L–1 SNP for 3 h inhibited germination, but treatment with the NO scavenger (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) to remove excess cellular NO promoted embryo germination, and facilitated chlorophyll synthesis and the accumulation of reactive oxygen species (ROS) (Yang et al. 2013). A low concentration of exogenous NO (2 mmol L–1) was found to increase the proportion and growth rate of germinating S. pohuashanensis embryos (Yang et al. 2018). However, the mechanism by which exogenous NO releases S. pohuashanensis seeds from dormancy is unclear.
On the basis of the above research, we studied the effects of the exogenous NO donor SNP, the NO scavenger PTIO, abscisic acid (ABA), the exogenous ethylene donor ethrel, and the ethylene receptor inhibitor 2,5-norbornadiene (NBD) on the germination of S. pohuashanensis embryos, the level of endogenous hormones, and ROS metabolism using a laboratory-based Petri dish germination method. The findings could be used to promote germination of S. pohuashanensis seeds, which could foster the protection and expansion of S. pohuashanensis natural resources.
Materials and methods
Experimental materials
S. pohuashanensis fruit were collected in late September at the Fenghuang mountain forest farm, Shanhetun Forestry Bureau in Wuchang City, Heilongjiang Province, China (east longitude 127° 28′ 18″–128° 14′ 36″, north latitude 44° 3′ 34″–44° 38′ 34″). Mature fruit harvested from a full-grown, healthy wild mature tree were kneaded by hand in water to remove the pericarp and pulp, seeds washed with water to remove any debris and air-dried at room temperature. Full dry seeds were stored in sealed plastic bags at 5 °C.
Experimental method
Seed pretreatment
Dormant seeds with full grain and uniform texture were selected (average weight per 1000 seeds, 1.95 g; average moisture content, 8.4%; average seed viability, 83.3%). Seeds were soaked in distilled water at 20 °C ± 5 °C for 48 h, further soaked in 0.2% (v/v) NaClO solution for 10–15 min, and rinsed with water. Seed coats were stripped on the ice (low temperature environment to avoid enzyme inactivation) and subsequent experiments were carried out with peeled embryos.
Embryo germination
The chemical reagents used for embryo treatment are shown in Table S1.
Pretreatment of embryos with SNP
Embryos were treated with SNP according to Bethke et al. (2006). A piece of filter paper was placed in a 500-mL beaker and dampened with 5 mL of 0.05 mol L–1 Hepes–KOH (pH 7.0). Ninety embryos were placed on the filter paper. A small beaker containing 5 mL freshly prepared SNP solution was placed in the 500-mL beaker, and the mouth sealed with plastic wrap (Fig. S1). Gaseous NO was released directly from the SNP solution and embryos exposed to the SNP vapour at 25 °C under 60 μmol m–2 s–1 light intensity for 3 h. After treatment, the embryos were washed with distilled water and placed on new filter paper wetted with distilled water. Controls were peeled embryos without SNP pretreatment. Control and pretreated embryos were germinated in 9-cm-diameter culture dishes at 25 °C under 60 μmol m–2 s–1 light intensity. The control and treatment experiments comprised three replicates.
Combined treatment of embryos with SNP and PTIO
After pretreatment with SNP, the embryos were placed on filter paper dampened with 3 mL 300 μmol L–1 PTIO solution. Control embryos were placed similarly. Germination conditions were the same as in pretreatment with SNP. Control and treatments experiments comprised three replicates.
Combined treatment of embryos with SNP and ABA
After pretreatment with SNP, the embryos were placed on filter paper dampened with 3 mL 3.0 μmol L–1 ABA solution. Control embryos were similarly placed on filter paper wetted with 3 mL of different ABA concentrations. Germination conditions were the same as in pretreatment with SNP. Control and treatments experiments comprised three replicates.
Combined treatment of embryos with SNP and NBD
After pretreatment with SNP, the embryos were placed on filter paper dampened with 3 mL 1 mmol L–1 NBD solution or with 3 mL 20 mmol L–1 ethrel. Control embryos were similarly placed on the filter paper wetted with 3 mL NBD or with ethrel solution. Germination conditions were the same as in pretreatment with SNP. Control and treatments experiments comprised three replicates.
Observing and recording germination
Germination was evident when the two cotyledons were green and the hypocotyl and radicle were elongated. Statistics were recorded for the time of germination initiation for the different treatments, germination percentage each day during the experiment, and cumulative germination percentage for different treatments.
Endogenous hormone assay
Embryos and seedlings cultured for 1–8 days were collected as test materials (0.5 g for each treatment) with three replicates. ABA level was assayed according to Ali-Rachedi et al. (2004) and ethylene level according to Gniazdowska et al. (2007).
ROS accumulation assay
Hydrogen peroxide (H2O2) level and superoxide anion were assayed according to Gniazdowska et al. (2010), and malondialdehyde (MDA) assayed according to Lu et al. (2009).
Antioxidant enzyme activity assay
Soluble protein content and superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) were determined according to Wang et al. (2017); glutathione peroxidase and glutathione (GSH-PX and GSH), respectively, according to Sousa et al. 2015).
Data statistics and analysis
Excel 2003 software (Microsoft) was used for data processing, and SPSS software (v17.0, SPSS Inc.) to analyze level differences of ROS, MDA, GSH and antioxidase, embryo germination percentage, radicle length, and ethylene production by ANOVA and Duncan’s multiple comparison and correlation analysis. Graphs were plotted using Sigmaplot software (v12.5, SYSTAT). Data concerning percentages were arcsine-transformed before analysis. All data represent the means of three replicates ± SD for each treatment. The effect of each influencing factor was evaluated by variance analysis, and a significance test performed at P = 0.05. Mean values were compared using the least significant difference test (Duncan’s) at the 5% level of significance.
Results
Germination of S. pohuashanensis embryos
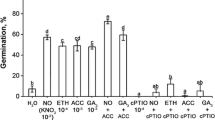
Treatment with exogenous chemicals significantly affected dormancy, germination, and growth of the embryos (Figs. 1a–i, 2a–f). Both the SNP and ethrel treatments significantly promoted embryo germination (Fig. 2a, c). By day 8, embryo germination percentage in the SNP and ethrel treatments was ˃ 75% (40% higher than in the controls), while those in the PTIO, NBD, and ABA treatments were only 43%, 29%, and 22%, respectively (19%, 45% and 58% lower than in the controls, respectively) (Fig. 2a, c, e). Pretreatment with SNP before the PTIO, NBD, or ABA treatments weakened but did not completely alleviate their inhibitory effects on embryo germination. The germination percentage in the SNP pretreatment was increased but it was still significantly lower than in the controls (Fig. 2a, c, e).
Germination of embryos and radicle growth of Sorbus pohuashanensis embryos; a, b SNP-treatment and (or) PTIO-treatment. a Embryo germination percentage; b radicle length 8 days after sowing. c, d ethrel-treatment or NBD-treatment or treatment of SNP in coordination with NBD, c embryo germination percentage, d radicle length 8 days after sowing, e, f treatments of ABA or SNP in coordination with ABA, e embryo germination percentage, f radicle length 8 days after sowing. Bars indicate means ± SE (n = 3). Different letters indicate significant differences at p = 0.05
SNP and ethrel also promoted radicle growth of newly germinated S. pohuashanensis seedlings. By day 8, average length of radicles in the SNP and ethrel treatments were 22% and 33%, respectively, greater than in the controls (Fig. 2b, d). PTIO, NBD, and ABA inhibited radicle growth with the average radicle length decreased by 52%, 86%, and 84%, respectively, compared to the controls (Fig. 2b, d, f). SNP pretreatment before treatments with PTIO, NBD, or ABA relieved the inhibitory effect of PTIO treatment on radicle growth (Fig. 2b), but the effects of NBD and ABA were still significant (Fig. 2d, f).
Changes in endogenous hormones during germination of S. pohuashanensis
In SNP-treated and ethrel-treated embryos, the levels of endogenous ethylene increased gradually from day 1–8 (by day 8, 37% and 42% greater, respectively, than the controls, Fig. 3a, b. Ethylene level was inhibited by treatments with PTIO, NBD, and ABA (Fig. 3a–c). Pretreatment with SNP before the PTIO treatment weakened its inhibitory effect but the results were not statistically significant (data not shown). Treatment with SNP or ethrel decreased endogenous ABA levels. Pretreatment with SNP (combined SNP and ABA) reduced endogenous ABA to 0.3 μg g–1 DW from day 5 onward. Treatment with NBD or the combination of SNP and NBD did not significantly affect endogenous ABA in embryos (0.81 μg g–1 DW).
Analysis of endogenous ROS accumulation in S. pohuashanensis embryos
In each treatment, H2O2 level increased during germination. Compared with the controls, SNP-treated embryos had significantly higher H2O2 levels and PTIO-treated embryos significantly lower (Fig. 4a). The levels of superoxide anions in SNP-treated embryos were lower than in the controls, while they were higher in PTIO-treated embryos (Fig. 4b).
The ROS levels in various organs responded differently to the treatments. The H2O2 level in radicles significantly increased in SNP-treated seedlings and significantly decreased in PTIO-treated seedlings (0.63 μg g–1 mL−1), while the superoxide levels decreased significantly to 7.33 μg g–1 mL−1 and 13.33 μg g–1 mL−1, respectively (Table 1).
Responses were also inconsistent between upper and lower cotyledons of seedlings. After SNP treatment, H2O2 levels (2.20 μg g–1 mL−1) significantly increased in lower cotyledons, while superoxide anion (7.00 O2− g−1 mL−1) significantly decreased in upper cotyledons. In the PTIO treatment, H2O2 level (0.97 μg g–1 mL−1) significantly decreased in upper cotyledons but significantly increased in the lower ones (1.37 μg g–1 mL−1), and superoxide anions levels were only slightly affected (Table 1). When embryos were pretreated with SNP (i.e., combined with SPN and PTIO), H2O2 levels in the upper cotyledons (0.92 μg g–1 mL−1) and lower cotyledons (1.31 μg g–1 mL−1) approached the levels in the PTIO treatment, and was not significantly different in the radicles (1.11 μg g–1 mL−1) from the controls. Levels of superoxide anions in the upper cotyledons (8.67 O2− g−1 mL−1) and radicles (9.33 O2− g−1 mL−1) significantly decreased in the SNP treatment (Table 1).
Malondialdehyde (MDA) levels decreased in SNP-treated embryos but increased significantly in ones treated with PTIO (Fig. 5); SNP pretreatment decreased the effect of the PTIO. This pattern of change was also observed in radicles and both upper and lower cotyledons (Table 1).
Effect of NO on antioxidant enzyme activities in S. pohuashanensis embryos
The activities of superoxide dismutase (SOD) and catalase (CAT), antioxidant enzymes that scavenge ROS in embryos, were increased significantly in SNP-treated embryos but decreased in PTIO-treated ones. SNP pretreatment alleviated the inhibitory effects of PTIO on SOD and CAT activities (Fig. 6a, b). The effects of these treatments on SOD and CAT activities were also detected in radicles and upper and lower cotyledons, with trends that were consistent with those detected in whole embryos (Table 2).
Glutathione (GSH) level (Fig. 7a) and GHS-peroxidase (GHS-PX) activity (Fig. 7b) increased significantly in SNP-treated embryos. PTIO did not affect GSH levels in embryos (Fig. 7a), but significantly reduced GSH-PX activity (Fig. 7b). SNP pretreatment before PTIO treatment weakened the inhibitory effect of PTIO on GHS-PX activity in embryos (Fig. 7b).
Compared to the controls, SNP-treated and PTIO-treated seedlings showed increased GSH levels in upper cotyledons, and significantly decreased GSH in lower cotyledons and radicles (Table 2). GSH-PX activity in all seedling organs increased significantly after SNP treatment and decreased significantly after PTIO treatment (Table 2). Similarly, SNP pretreatment before PTIO treatment weakened its effect on GSH level in lower cotyledons and GHS-PX activity in upper and lower cotyledons (Table 2).
Means followed by different lowercase letters are significantly different according to Duncan’s -test (P < 0.05) (mean ± SD; n = 3).
Discussion
Roles of NO and H 2 O 2 in seed dormancy release and germination
Seed dormancy is an adaptive mechanism of plants to environmental change (Holdsworth et al. 2008; Su et al. 2016). The ability to germinate is regulated by combination of environmental and endogenous signals with both synergistic and antagonistic effects (Arc et al. 2013). Some previous studies have focused on the relationship between hormonal regulation and the breaking of seed dormancy (Yang et al. 2009; Deng et al. 2016; Ma et al. 2018). There is experimental evidence that nitric oxide can break dormancy (Bethke et al. 2007; Basson-Bard et al. 2008; Arc et al. 2013), and that this is related to changes in reactive oxygen species in the seeds (Gniazdowska et al. 2010; Arc et al. 2013; Yang et al. 2013, 2018). In this study, we found that NO and ROS are involved in embryo germination and seedling development, consistent with the proposed role of NO as an important inducer of seed dormancy release (Arc et al. 2013).
In the present study, after 8 days of SNP treatment, germination of embryos was > 90% (Fig. 2a), and seedlings also showed the fastest radicle growth (Fig. 2b). However, germination and radicle elongation were significantly reduced after treatment with the NO scavenger PTIO (Fig. 2a, b). SNP treatment increased H2O2 concentration in S. pohuashanensis embryos (Fig. 4a). Moreover, variations in H2O2 concentration were related to variations in germination percentage (96%). (Fig. S2). These results demonstrate that NO and ROS are involved in the release of embryo dormancy and subsequent germination, and in seedling development of S. pohuashanensis. This is consistent with results reported for embryos of other species such as apple (Malus domestica; Gniazdowska et al. 2010) and empress tree (Paulownia tomentosa (Thunb.) Steud.; Giba et al. 1998).
Germination is a complex physiological and biochemical process mainly controlled by the abscisic acid concentration or by the ratio (or balance) of ABA to GA (Nambara and Marion-Poll 2003; Pérez-Flores et al. 2003; Finch-Savage and Leubner-Metzger 2006). There are two mechanisms by which NO breaks S. pohuashanensis embryo dormancy and promotes germination. First, as a signal molecule, nitric oxide can promote ABA catabolism (Matakiadis et al. 2009; Arc et al. 2013) to release dormancy. ABA can significantly inhibit germination of S. pohuashanensis embryos (Fig. 2e). In Arabidopsis, NO upregulates the expression of CYP707A2 and promotes ABA catabolism to reduce its concentration in seeds, or reduce the sensitivity of seeds to ABA (Liu et al. 2009a). Secondly, NO may interact with H2O2 to promote ABA catabolism, thereby breaking seed dormancy and promoting germination. Several lines of evidence support this; first, it was observed that exogenous SNP results in an increase in H2O2 in S. pohuashanensis embryos (Fig. 4a). Increased H2O2 levels promote the synthesis of endogenous NO, thus accelerating ABA catabolism (Bethke et al. 2007; Liu et al. 2009b, 2010a); secondly, with the participation of NO, H2O2 can upregulate genes encoding ABA 8′-hydroxylase (e.g., CYP707A2) (Liu et al. 2010a, b), leading to a significant decrease in ABA in embryos; thirdly, H2O2 can upregulate GA biosynthesis genes (e.g., GA3ox, GAw20ox) to increase biosynthesis and regulate the ratio of GA and ABA, which is also conducive to dormancy breaking and germination (Liu et al. 2010a, b).
Roles of SNP, H 2 O 2 , and antioxidant enzymes in seed dormancy release and germination
Our results show that the breaking of dormancy in S. pohuashanensis embryos is closely related to increased activities of antioxidant enzymes. Embryos treated with SNP accumulated H2O2 (Fig. 4a). This did not damage embryo cells (i.e., the MDA level was relatively low, Fig. 5) but promoted germination (Fig. S2). This may be explained by the regulation of ROS homeostasis by NO (Corpas et al. 2011; Ahmad et al. 2019; Fig. 4b). As demonstrated in our study, a low concentration of NO not only increases GSH level (Fig. 7a), but also increases SOD, CAT (Fig. 6a, b) and GSH-PX activities (Fig. 7b). With increased concentrations of H2O2 and superoxide (Fig. S3), the activities of SOD and CAT increase significantly to reduce ROS damage (Hu et al. 2007; Arc et al. 2013; Ishibashi et al. 2017). Previous studies have demonstrated that NO can react with reduced GSH or thiol groups, leading to the reversible formation of S-nitrosothiols (e.g., GSNO, S-nitrosylatedproteins). GSNO is a storage and transport form for NO in seeds (Catusse et al. 2008). NO-based modifications of antioxidant enzymes play a key role in the regulation of oxidative stress and in ROS homeostasis (Yang et al. 2015). Although our results show that antioxidant enzymes are involved in RNS-dependent dormancy release, the specific details of the NO-regulated ROS homeostasis mechanism need to be further studied.
Roles of SNP and hormones (ethylene) in seed dormancy release and germination
We also studied the interactions (crosstalk) between hormonal signals and NO-regulated ROS accumulation in seed dormancy and germination. Interestingly, in this study, exogenous ethylene as well as SNP broke dormancy and promoted germination of S. pohuashanensis embryos (Fig. 2c). Germination percent and radicle elongation were decreased by the ethylene receptor inhibitor NBD (Fig. 2c). Furthermore, we found that the NO signal led to an increase in H2O2, which was related to an increase in ethylene biosynthesis and a decrease in ABA during breaking of embryo dormancy. Ethylene decreased in embryos treated with the NO scavenger PTIO (Fig. 3a) and in those treated with the ethylene receptor inhibitor NBD (Fig. 3b). The ethylene level was positively correlated significantly with levels of H2O2 and superoxide, and the levels of all three were positively correlated significantly with percent germination of S. pohuashanensis embryos (see Figs. S2 and S4). These results demonstrate that NO participates in the crosstalk between ABA and ethylene through ROS signals to regulate dormancy and germination of S. pohuashanensis embryos.
Our results indicate that this NO-mediated release of dormancy depends on ethylene biosynthesis and signalling, which counteract the effect of ABA. This conclusion is consistent with findings for Arabidopsis seeds (Arc et al. 2013). Previous studies have demonstrated that the NO-induced ABA decrease is correlated with the regulation of CYP707A2 transcription and (+)-ABA 8′-hydroxylase (encoded by CYP707A2) protein expression (Liu et al. 2009a; Ma et al. 2018). A recent study showed that the ethylene receptors ETR1, EIN4, and ETR2 control ABA signalling during Arabidopsis seed germination (Bakshi et al. 2018). Yu et al. (2019) reported that ethylene and ABA antagonistically regulate ascorbic acid biosynthesis and ROS accumulation via ETHYLENE-INSENSITIVE3 (EIN3) and ABA INSENSITIVE4 (ABI4), key factors in the ethylene and ABA signalling pathways, respectively. However, it is still largely unknown whether or how ethylene affects GA biosynthesis and signalling during seed dormancy and germination.
Conclusions
This study focused on the roles and correlations among reactive nitrogen species (RNS), reactive oxygen species (ROS), and plant hormones in seed dormancy. The results show that nitric oxide breaks seed dormancy of S. pohuashanensis and is closely related to ethylene biosynthesis and abscisic acid catabolism. This signalling pathway is closely related to ROS accumulation and the antioxidant defence response. These results shed new light on the positive role of NO, ROS, and their interactions with plant hormones in germination of S. pohuashanensis.
References
Ahmad P, Tripathi DK, Deshmukh R, Singh VP, Corpas FJ (2019) Revisiting the role of ROS and RNS in plants. Environ Exp Bot 161:1–3
Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M (2004) Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219:479–488
Arc E, Sechet J, Corbineau F, Rajjou L, Marionpoll A (2013) ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4:63
Bakshi A, Piya S, Fernandez JC, Chervin C, Hewezi T, Binder B (2018) Ethylene receptors signal via a non-canonical pathway to regulate abscisic acid responses. Plant Physiol 176:910–929
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Bethke PC, Libourel IGL, Reinohl V, Jones RL (2006) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223:805–812
Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143:1173–1188
Bian L, Yang L, Wang JN, Shen HL (2013) Effects of KNO3 pretreatment and temperature on seed germination of Sorbus pohuashanensis. J For Res 24:309–316
Catusse J, Strub JM, Job C, Van Dorsselaer A, Jo D (2008) Proteome-wide characterization of sugarbeet seed vigor and its tissue specific expression. Proc Natl Acad Sci USA 105:10262–10267
Chaudhuri A, Singh K, Kar R (2013) Interaction of hormones with reactive oxygen species in regulating seed germination of Vigna radiata (L.) Wilczek. J Plant Bioch Physiol 1:1–5
Corpas FJ, Leterrier M, Valder-rama R, Airaki M, Chaki M, Palma JM, Barroso JB (2011) Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 181:604–611
Deng Z, Hu XF, Ai XR, Yao L, Deng SM, Pu X, Song SQ (2016) Dormancy release of Cotinus coggygria seeds under a precold moist stratification: an endogenous abscisic acid/gibberellic acid and comparative proteomic analysis. New For 47:105–118
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Giba Z, Grubisic D, Todorovic S, Sajc L, Stojakovic D, Konjevic R (1998) Effect of nitric oxide-releasing compounds on phytochrome-controlled germination of Empress tree seeds. Plant Growth Reg 26:175–181
Giba Z, Grubišić D, Konjević R (2007) Seeking the role of NO in breaking seed dormancy. Plant Cell Monogr 25(6):91–111
Gniazdowska A, Dobrzyjska U, Babajczyk T, Bogatek R (2007) Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 225:1051–1057
Gniazdowska A, Krasuska U, Czajkowska K, Bogatek R (2010) Nitric oxide, hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul 61:75–84
Holdsworth R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415
Hu KD, Hu LY, Li YH, Zhang FQ, Zhang H (2007) Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul 53:173–183
Ishibashi Y, Aoki N, Kasa S, Sakamoto M, Kai K, Tomokiyo R, Watabe G, Yuasa T, Iwaya-Inoue M (2017) The interrelationship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination. Front Plant Sci 8:1–10
Liu Y, Zhang J (2009) Rapid accumulation of NO regulates ABA catabolism and seed dormancy during imbibition in Arabidopsis. Plant Signal Behav 4(9):905–907
Liu YG, Shi L, Ye NH, Liu R, Jia WS, Zhang JH (2009) Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 183:1030–1042
Liu X, Deng Z, Cheng H, He X, Song S (2010a) Nitrite, sodium nitroprusside, potassium ferricyanide and hydrogen peroxide release dormancy of Amaranthus retroflexus seeds in a nitric oxide-dependent manner. Plant Growth Reg 64:155–161
Liu YG, Ye NH, Liu R, Chen MX, Zhang JH (2010b) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61(11):2979–2990
Lu JY, Xue H, Pan Y, Kan S, Liu M, Nechitailo GS (2009) Effect of spaceflight duration of subcellular morphologies and defense enzyme activities in earth-grown tomato seedlings propagated from space-flown seeds. Russ J Physiol Chem B 3:981–986
Ma YM, Chen XD, Guo BL (2018) Identification of genes involved in metabolism and signaling of abscisic acid and gibberellins during Epimedium pseudowushanense B.L. Guo seed morphophysiological dormancy. Plant Cell Rep 37:1061–1075
Malá J, Máchová P, Cvrčková H, Karady M, Novák O, Mikulík J, Hauserová E, Greplová J, Strnad M, Doležal K (2009) Micropropagation of wild service tree (Sorbus torminalis [L.] Crantz): the regulative role of different aromatic cytokinins during organogenesis. J Plant Growth Regul 28:341–348
Matakiadis T, Alboresi A, Jiku-maru Y, Tatematsu K, Pichon O, Renou JP, Kamiya YJ, Nambara E, Truong HN (2009) The Ara-bidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol 149:949–960
Nambara E, Marion-Poll A (2003) ABA action and interactions in seeds. Trends Plant Sci 8:213–217
Pérez-Flores L, Carrari F, Osuna-Fernández R, Rodríguez MV, Encisco S, Stanelloni R, Sánchez RA, Bottini R, Iusem ND, Benech-Arnold RL (2003) Expression analysis of a GA 20-oxidase in embryos from two sorghum lines with contrasting dormancy: possible participation of this gene in the hormonal control of germination. J Exp Bot 54(390):2071–2079
Sasaki K, Kishitani S, Abe F, Sato T (2015) Promotion of seedling growth of seeds of rice (Oryza sativa L. cv. Hitomebore) by treatment with H2O2 before sowing. Plant Prod Sci 8:509–514
Sousa RH, Carvalho FE, Ribeiro CW, Passaia G, Cunha JR, Lima-Melo Y, Margis-Pinheiro M, Silveira JA (2015) Peroxisomal APX knockdown triggers antioxidant mechanisms favourable for coping with high photorespiratory H2O2 induced by CAT deficiency in rice. Plant Cell Environ 38:499–513
Su LQ, Lan QY, Pritchard HW, Xue H, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Biochem 109:406–415
Wang X, Ma R, Cui D, Cao Q, Shan Z, Jiao Z (2017) Physio-biochemical and molecular mechanism underlying the enhanced heavy metal tolerance in highland barley seedlings pre-treated with low-dose gamma irradiation. Sci Rep-UK 7:14233
Xu JW, Shen HL, Zhang XL, Zhang P, Huang J (2010) Sorbus pohuashanensis seed dispersal and germination and their relationships with population natural regeneration. Chin J Appl Ecol 21:2536–2544 ((in Chinese))
Yang L, Shen HL (2011) Effect of electrostatic field on seed germination and seedling growth of Sorbus pohuashanesis. J For Res 22:27–34
Yang L, Liu CP, Shen HL (2008a) Effect of cold stratification durationsand germination temperatures on seed germination of Sorbus pohuashanensis. Seed 27(20–23):25 ((in Chinese))
Yang L, Shen HL, Liang LD, Liu CP (2008b) Changes in endogenous hormone content in seeds of Sorbus pohuashanensis (Hance) Hedl during artificial drying and cold stratification. Plant Physiol Com 44:682–688 ((in Chinese))
Yang L, Cui XT, Shen HL (2009) Effects of exogenous plant growth substances and temperature on seed germination of Sorbus pohuashanensis (Hance) Hedl. Plant Physiol Commun 45(6):555–560 ((in Chinese))
Yang L, Li YH, Shen HL (2012a) Somatic embryogenesis and plant regeneration from immature zygotic embryo cultures of mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Org 109:547–556
Yang L, Wang JN, Bian L, Li YH, Shen HL (2012b) Cyclic secondary somatic embryogenesis and efficient plant regeneration in mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Org 111:173–182
Yang L, Wang JN, Bian L, Shen HL (2013) Effects of exogenous nitric oxide on embryo germination and ROS accumulation in seedling growth initial stage of Sorbus pohuashanensis. Sci Sil Sin 49:60–67 ((in Chinese))
Yang HJ, Mu JY, Chen LC, Feng J, Hu JL, Li L, Zhou JM, Zuo JR (2015) S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol 167:1604–1615
Yang L, Zhang DY, Liu HN, Wei C, Wang JN, Shen HL (2018) Effects of a nitric oxide donor and nitric oxide scavengers on Sorbus pohuashanensis embryo germination. J Forest Res 29(3):631–638
Yu YW, Wang J, Li SH, Kakan X, Zhou Y, Miao YC, Wang FF, Qin H, Huang RF (2019) Ascorbic acid integrates the antagonistic modulation of ethylene and abscisic acid in the accumulation of reactive oxygen species. Plant Physiol 179:1861–1875
Acknowledgements
The authors thank Dr. Zhengquan Wang from the Northeast Forestry University China for comments that improved an earlier draft of this paper, and also two anonymous reviewers and the editor for comments.
Author information
Authors and Affiliations
Contributions
Y. L and S. HL conceived and designed the study. W. JN collected plant materials and prepared samples for analysis. W. H and W. JN analyzed the results for experiments. Y. L, W. H and T. SR contributed to the writing of the manuscript and data analyses. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was provided by National Natural Science Foundation of China (No. 32071757) and National Natural Science Foundation of Heilongjiang Province of China (C201407).
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Tang, S., Wang, J. et al. Interaction between reactive oxygen species and hormones during the breaking of embryo dormancy in Sorbus pohuashanensis by exogenous nitric oxide. J. For. Res. 33, 435–444 (2022). https://doi.org/10.1007/s11676-021-01330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01330-y