Abstract
In northeastern Japan, an area of high precipitation and mountains, beech (Fagus creanata Blume), larch (Larix kaempferi Lamb.), cedar (Cryptomeria japonica D. Don) and black locust (Robinia pseudoacacia L.) were evaluated for N resorption and N isotope fractionation in pre- and post-abscission leaves in comparison to green leaves. The highest leaf N concentration in summer corresponded to the N-fixing black locust, followed in decreasing order by the deciduous beech and larch and evergreen cedar. On the other hand, the lowest N resorption efficiency corresponded to black locust and the highest to beech, in increasing order by larch and cedar. All tree species returned significant amounts of N before leaf abscission; however, N isotope fractionation during leaf N resorption was only found for beech, with a depleted N isotope value from green to pre-abscission leaf. The most N, however, was resorbed from pre-abscission to post-abscission. This result may indicate that δ15N fractionation took place during N transformation processes, such as protein hydrolysis, when the concentration of free amino acids increased sharply. The difference in the type of amino acid produced by each species could have influenced the N isotope ratio in beech but not in the other tree species. The results of this study showed that it is possible to infer the type and timing of processes relevant to N resorption by analyzing leaf δ15N variation during senescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen is one of the main elements necessary for forest ecosystem growth. However, its acquisition is limited by N mineralization and N loss through processes such as leaching and volatilization (Vergutz et al. 2012). Therefore, a key function for plants is the retention of nutrients via resorption from senescing leaves at the end of the growing season. Plants generally reabsorb half of their total leaf N, yet there is large interspecific variation in the extent of N resorption (Aerts 1996; Yuan and Chen 2009). The stored N is reallocated mainly to the stem and roots for new growing tissues the next spring (Millard 1994; van Heerwaarden et al. 2003; Singh 2014, 2015).
N resorption reflects plant dependence on soil N supply, with leguminous species having a lower N resorption efficiency (NRE) (Singh 2014, 2015). The resorbed N is stored in plant tissues after leaf senescence (van Heerwaarden et al. 2003; Vergutz et al. 2012; Chavez-Vergara et al. 2015); thus, deciduous trees having a significantly higher NRE than evergreen trees (Aerts 1996; Yuan and Chen 2009). Previous studies have also shown that N resorption proficiency (NRP) (N content per dry leaf mass in senescing leaves) is a useful parameter for quantifying the amount of actual N that returns to storage tissues (Wright and Westoby 2003; Lima et al. 2006; Özbucak et al. 2008; Chavez-Vergara et al. 2015). In mixed forests, tree species with lower NRE contribute more N in the leaf litter, which thus increases N availability in the soil (Lopez et al. 2014; Chavez-Vergara et al. 2015) and provides N to neighboring plants. Even though many studies have dealt with the process of resorption, very few studies have used isotope analysis to examine N isotope discrimination during this process (Kolb and Evans 2002; Templer et al. 2007), especially in the context of the duration of resorption. The use of stable isotopes is relevant because of the effect of free amino acids release during protein hydrolysis (Chapin and Kedrowski 1983; Vitousek et al. 1989) on foliar δ15N as the concentration of free amino acids in leaves increase (Killingbeck 2004; Mae 2004) during the first stage of N withdrawal.
Foliar δ15N varies by over 32‰ among plants found in natural ecosystems (Yoneyama et al. 1993; Aranibar et al. 2004; Codron et al. 2005; Hobbie et al. 2005) and systematically along gradients of climate and nutrient cycling (Amundson et al. 2003; Pardo et al. 2006; Craine et al. 2009) and among species (Michelsen et al. 1996; Hobbie et al. 2005; Templer et al. 2007; Kahmen et al. 2008). Differences during the growing season have been reported (Handley et al. 1999), but other studies found no difference during that period (Pardo et al. 2006, 2013). Discrimination of 15N could potentially occur during either the biochemical processes involved in N resorption from senescing leaves and thus cloud interpretations of community-wide patterns of foliar δ15N. Amino acids are the primary transport form of N, but as N mostly occurs in proteins and chlorophylls, biochemical processes are needed to convert the N to transportable forms (Fotelli et al. 2002; Ueda et al. 2011). Protein hydrolysis, like many biochemical reactions, may involve discrimination against 15N, which could lead to variation in the isotopic composition of different plant tissues (Shearer and Kohl 1989; Babst and Coleman 2018), which would be also expected during chlorophyll degradation.
The present study addresses whether N discrimination occurs during leaf N resorption by examining patterns of δ15N prior and during leaf senescence in four types of functional groups of trees that are typical for Japan: deciduous (Fagus crenata Blume; Fagaceae), deciduous–coniferous (Larix kaempferi Lamb.; Laraceae), evergreen (Cryptomeria japonica D. Don) and invasive legume (Robinia pseudoacacia L.; Leguminosae).
Many of the studies on N resorption have focused on comparing the leaf N concentration between mature and abscised leaves (Negi and Singh 1993; Özbucak et al. 2008; Chavez-Vergara et al. 2015), but very few have focused on the duration of this resorption (Chapin and Kedrowski 1983; Wang et al. 2013). This study aimed to clarify the response of the N cycle in representative species in a heavy rainfall area, such as northeastern Japan, by focusing on the amount of N being returned to trees or input in the soil as litter and by tracing stable isotopes in the N fraction that is recycled within the forest ecosystem. We, thus compared the NRE of the four tree species and evaluated the effects of N transformation processes on N isotope discrimination during resorption.
Materials and methods
Study area
This study was conducted at the Yamagata University Research Forest, Japan (38° 33ʹ N, 139° 51ʹ E) (Fig. 1) in the northernmost area of the Asahi Mountains, a cool temperate deciduous broad-leaved forest zone. The annual mean air temperature is 13.4°C, mean annual precipitation is 2500 mm, and mean snow depth is approximately 3 m.
Four types of functional groups typical in this region were investigated: black locust (R. pseudoacacia; Leguminosae), deciduous beech (F. crenata; Fagaceae), larch (L. kaempferi; Pinaceae) and cedar (C. japonica; Cupressaceae) were sampled in forest stands that were close to each other (Fig. 1); each species was dominant in its stand. The invasive black locust stand invaded a former cedar forest site that was cut and cleared by slash and burn in 2011. The beech stand is natural for this area and most common, followed by the cedar plantations. The age of the sampled larch and cedar plantations was around 60 years, similar to the beech stand, while the black locust stand was the youngest at 7 years.
Meteorological conditions
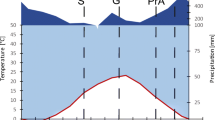
The meteorological station in the Yamagata University Research Forest is located near the sampled sites (Fig. 1). During each month from June to November in 2016, at least 100 mm precipitation fell, with 47% of the precipitation in July and August, when air temperatures (Ta) was the highest. Precipitation then decreased until January 2017 (Fig. 2).
Sampling
The leaf and needle samples for the selected tree species were collected three times between August and November 2016. The first sampling took place during the first week of August when maximum growth was reached, as indicated by installed dendrometers, and the maximum mass N pool sizes are expected (Chapin and Kedrowski 1983). This period is defined as green leaf, while the second sampling period, defined as pre-abscission, took place in mid-October when more than 10% of the deciduous trees leaves were starting to change colors. The post-abscission samples were collected by mid-November, assuming that leaching between leaf fall and leaf collection was negligible (Vergutz et al. 2012). The leaf samples in August and October were collected randomly from eight cedar and eight black locust trees and from six larch and six beech trees. All leaf samples were selected to avoid leaves that were damaged by insects (Özbucak et al. 2008) and taken from branches that were at a reachable height. To collect the post-abscission leaf samples, three 80 × 80 cm litter traps were set up in each of the four stands from the second week of September until the second week of November. Leaf samples of cedar were not divided by leaf age groups because N concentrations were previously found not to differ among ages (Kobayashi and Tashiro 2003).
Analysis
N concentration (%N) and δ15N (‰)
All leaf samples were oven dried at 70°C for 48 h, ground, then N concentration and δ15N were measured with a CF-IRMS (continuous-flow type mass spectrometer: Iso Prime mass spectrometer, GV Instruments, Withenshave, UK). The evolved gas was first passed through a column of packed solid CaO–NaOH (soda lime) to eliminate excess CO2 and then introduced into a gas chromatograph to separate N2, 15N/14N ratios were determined with the mass spectrometer. Analytical errors associated with the overall procedure for δ15N measurement are less than 0.2‰. The results of δ15N were expressed as ‰ deviation, relative to atmospheric N2 (δ15N = 0‰):
Nitrogen resorption efficiency (NRE) and resorption proficiency (NRP)
NRE is calculated as the difference between the N concentration in the green leaves and the N concentration in leaves after abscission divided by the N concentration in green leaves, and is expressed as a percentage:
In addition, N resorption efficiency was corrected according to method of Vergutz et al. (2012) (NRE*) by multiplying NRE by the mass loss correction factor of 0.754 for conifers and 0.784 for deciduous angiosperms (Vergutz et al. 2012), because loss in leaf mass during senescence can lead to an underestimation of 10% (van Heerwaarden et al. 2003).
NRP, that is, N concentration per leaf mass in senescing leaves (in % dry mass), is used as a more stable indicator of plant ability to recycle nutrients than NRE (Killingbeck 2004; Yasumura et al. 2005; Yuan et al. 2005). Leaves that can reduce N concentration to a lower level are more proficient in resorbing N.
Amino acid content
All samples were oven dried at 70 °C for 48 h, ground, and analyzed for amino acid content by capillary electrophoresis and mass spectrometry (CE-TOFMS, CE type, TOF/MS 6224, Agilent Technologies). The sample extraction, preparation and CE/MS analysis were done using the methods of Oikawa et al. (2015). Three samples for each period (green leaf, pre-abscission and post-abscission) were analyzed.
Statistical analyses
The data were subjected to analysis of variance (ANOVA) and a two-way ANOVA (Statcel 2.0, OMS, Tokyo, Japan) to compare the effects of different sample periods and tree species. Changes of all measured parameters in leaves and needles along the three sampling times within one species were compared first (n = 8 for cedar and black locust, n = 6 for larch and beech) followed by a comparison of leaf and needle values along sampling times between species. The significant difference was determined with the Tukey–Kramer test (p < 0.05).
Results
Nitrogen concentration, NRE and NRP
All investigated species exhibited different trends in %N from green leaf to post-abscission, with the %N response of a species affected by a significant species by phenological date interaction (F6, 32 = 6.75; p < 0.001). First, %N in beech had a significant decreasing trend across the phenological stages, from green to pre-abscission (19.8%) and from pre- to post-abscission (48.8%). Larch and cedar had a similar pattern but with no significant decrease from green leaf (1.7 ± 0.3%N and 1.4 ± 01%N) to pre-abscission (1.4 ± 0.3%N and 1.3 ± 0.2%N). However, the %N was significantly lower in the post-abscission period (0.8 ± 0.0%N and 0.8 ± 0.2%N). Finally, black locust had greater % N than in the other species; although the level remained higher across the phenological stages, it had non-significant decreases from green to pre-abscission and from pre- to post-abscission leaves (10.3% and 7.7%, respectively).
All investigated species had a continuous decrease in %N from green leaf to post-abscission. Beech and larch N concentration had no significant difference in mean %N for the overall period, while the leaf N concentration in black locust was more than double the concentration in larch and cedar (Fig. 3).
The %N in beech decreased significantly among the sampling periods and was the only tree species with a significant decrease in N concentration for each period (19.8% decrease from green to pre-abscission, 48.8% from pre- to post-abscission). Larch and cedar had a similar pattern but the decrease was not significant from green leaf (1.7 ± 0.3%N and 1.4 ± 01%N) to pre-abscission (1.4 ± 0.3%N and 1.3 ± 0.2%N), but %N was significantly lower in the post-abscission period (0.8 ± 0.0%N and 0.8 ± 0.2%N). For black locust, N concentration from green to pre-abscission decreased by 10.3% and from pre- to post-abscission leaves by 7.7%.
Beech leaves had the highest NRE (68.7%) among the four species, black locust the lowest (18.0%). The NRE of larch (50.9%) was slightly higher than cedar (42.6%) and in-between the values for beech and black locust. Correspondingly, the NRP was the highest (= least proficient) for black locust followed by larch and cedar trees and low values for beech trees (Table 1). The NRE and NRE* showed the same significant differences between beech and locust and the other species, while no difference was found between larch and cedar. In the case of NRP there was no significant difference among beech, larch and cedar but black locust was significantly different to all the other tree species.
Leaf amino acids
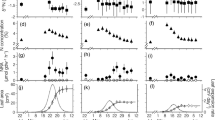
The total amino acid content in beech, larch and black locust significantly increased from green leaves (0.8 ± 0.3 mg g−1, 0.2 ± 0.1 mg g−1 and 0.2 ± 0.1 mg g−1 respectively) to pre-abscission leaves (3.2 ± 0.4 mg g−1, 2.1 ± 0.3 mg g−1 and 3.2 ± 0.9 mg g−1, respectively), then decreased significantly in post abscission leaves (1.1 ± 0.5 mg g−1, 0.1 ± 0.0 mg g−1 and 0.7 ± 0.3 mg g−1 respectively). The main amino acids that increased, but to a different extent for these three species, were alanine (Ala), asparagine (Asn), glutamine (Gln), isoleucine (Ile), leucine (Leu) and valine (Val). The level of most amino acids in cedar leaves did not differ significantly during the growing season; only arginine (Arg), Asn, glutamic acid (Glu) and tryptophan (Trp) varied significantly (Fig. 4).
Leaf δ15N at different growth periods
Beech was the only species in which leaf δ15N was significantly higher at the green leaf period (− 1.5 ± 0.5‰) than at the pre- (− 2.6 ± 0.3‰) and at the post-abscission (− 2.7 ± 0.4‰) periods, but did not differ significantly between the pre- and post-abscission. Although larch leaf δ15N was lower at each consecutive sampling (0.5 ± 1.0‰ to 0.0 ± 1.1‰ to − 0.9 ± 0.1‰), differences among periods were not significant. Cedar and black locust each only changed slightly in leaf δ15N among the periods (cedar was slightly depleted: − 0.4‰ from green leaf [− 2.2 ± 0.5‰] to post-abscission stage [− 2.6 ± 0.5‰]; black locust was slightly enriched: 0.2‰ from green leaf [0.0 ± 0.3‰] to pre-abscission [0.2 ± 0.5‰]) (Fig. 5). The statistical analysis showed that 15N response was species specific with a significant species by sampling date interaction (F6, 32 = 7.75; p < 0.0001).
Leaf δ15N for all non-leguminous tree species decreased with time, and was slightly enriched in black locust. The average value for the whole period in beech and cedar were depleted and in a similar range, while larch and black locust leaves were significantly enriched.
Discussion
N concentration, NRE and NRP
The decrease in N concentration from green to pre-abscission leaves was greatest in beech trees. In our study, NRE for beech was 68.7%, higher than that for beech trees in Spain (59.4%) (Santa Regina and Tarazona 2001), but agreed with findings of Vergutz et al. (2012) who reported a decrease in NRE with latitude, mean annual temperature (MAT) and mean annual precipitation (MAP). Beech NRE* in our study was 11.2% higher than in deciduous angiosperms in a global comparison of different species in different climate zones (Vergutz et al. 2012), which could be due to the high MAP (3000 mm) in our study area. Similarly, Yasumura et al. (2005) found NRE values of nearly 70% for beech (F. crenata) in northern Japan. The green leaf N concentration in beech in our study was similar to that found by Tateno and Takeda (2010) for beech in Japan (20.7 mg g−1).
The green leaf N concentration for larch was not significantly different from that of beech, but N resorption took place over a shorter period than for beech, mainly from October to November. NRE* of larch was in the same range for conifers (Vergutz et al. 2012), while for the evergreen coniferous cedar, the leaf N concentration decrease was similar to that found in larch leaves, but the NRE* was lower. A reason might be that cedar needles were not separated by age and were pooled together; however, Kobayashi and Tashiro (2003) reported values (N content: 16.7 mg g−1 for, NRE: 49.1%) for cedar in Japan that were similar to those in our study. Despite the high differences in the NRE for the three non-leguminous species, NRP values did not differ significantly. Therefore, the combined use of NRE and NRP gives a better overall understanding of foliar N movement during the growing season.
Black locust had significantly higher foliar N concentration than the other tree species throughout the growing season. The N concentration in black locust of green, pre- and post-abscission leaves was also high compared to results for pre- (1.9–2.3%N) and post-abscission (1.1–1.5%N) (Singh 2014, 2015) and the global average for N-fixing deciduous angiosperms for green (2.4%N) and post-abscission leaf (1.5%N) (Vergutz et al. 2012). In comparison to the other non-leguminous tree species, the NRE was lower, which agrees with previous studies (Lima et al. 2006; Singh 2014). However, the NRE found in our study was lower than in previous studies, which could be due to the tropical environment of the trees in the other studies. In addition, the same species has a lower potential for N resorption in colder environment than in warmer ones (González-Zurdo et al. 2015; Sun et al. 2016). The establishment of black locust in this site, with its low NRE, changed the soil N characteristics by increasing soil fertility in comparison to the other tree species, as found previously (Chavez-Vergara et al. 2014).
δ15N values and amino acids
Comparison among tree species
Leaf δ15N values differed among the four tree species, with leaf δ15N depleted most in beech, and enriched most in larch. The significant enrichment in larch, compared to the other non-leguminous tree species, may originate from the preferential type of N uptake from the soil, which is generally enriched in ammonium compared to nitrate. In this study, the main form of inorganic N is ammonium (Seidel et al. 2017), which is also the preferred N type for larch (Malagoli et al. 2000). Another influence on the δ15N value in the leaves could be mycorrhizal associations of the tree species; plants associated with ectomycorrhizal fungi (ECM) such as beech and larch have depleted δ15N compared to those with arbuscular mycorrhizal fungi (AM) (Michelsen et al. 1996; Schmidt and Stewart 2003) such as cedar. However, larch leaves were in general substantially more enriched in δ15N compared to the other two species, which could indicate a lower reliance on ECM during N uptake (Hayashi et al. 2018). Differences in leaf δ15N among tree species could also be due to differences in rooting depth, although beech and larch are characterized by shallower rooting, or physiological factors such as different leaf-out dates, which might give one species access to a different N pool, but that seems unlikely. Therefore, the enriched δ15N values for larch seemed to be due mainly to preferential uptake of ammonium and physiological differences in transport, and assimilation of N between these species (Pardo et al. 2006, 2013), because leaf δ15N values in the present study agree with values found in larch forests of Mongolia (Hayashi et al. 2018). Leaf δ15N in black locust and cedar were similar to tree-ring δ15N values of black locust (close to atmospheric δ15N = 0) and black pine in coastal areas (ranging from − 2.0 to − 4.0‰) of the Shonai Region (Lopez et al. 2014), respectively.
δ15N during the growing season
The non-N-fixing tree species showed a decreasing trend in δ15N during the growing season, but except for beech, there were no significant differences during N mobilization from senescent leaves to storage organs, despite significant leaf N resorption before leaf abscission in all of the tree species. In previous studies, no fractionation of δ15N was found during resorption for various deciduous species (Kolb and Evans 2002; Templer et al. 2007), although these studies did not disprove that this result might be species dependent. Pardo et al. (2013) also found no significant difference between leaf δ15N during the growing season and fresh litter δ15N, while Handley et al. (1999) and Näsholm (1994) reported variation in foliar δ15N during the growing season.
In the two early periods, the highest percentage of N was lost in beech, with N fractionation from the green to the pre-abscission leaf δ15N, but not from the pre- to the post-abscission leaves. In Scots pine, Näsholm (1994) also found a significant enrichment from green needle to senescent needle, which he hypothesized was due to ammonia volatilization during senescence. This possibility seems unlikely in the present study because leaf δ15N decreased from green leaf to pre-abscission, and not during the main period of senescence. Adriaenssens et al. (2012) found for beech the highest uptake of N via the air (δ15N = 0‰) in August, which could contribute to an enriched leaf δ15N values compared to the following months.
For our study, asparagine was the amino acid that increased most from green to pre-abscission leaves in beech (140 times), and thus may have contributed to the lower isotopic values in pre-abscised leaves because it has less δ15N compared to other primary amino acids (Gauthier et al. 2013). Gauthier et al. (2013) found for rapeseed leaves that the free amino acids exhibited high fractionation during enzymatic reactions in primary N metabolism. Thus, the N isotope fractionation in beech leaves could be the result of one or more of the above mentioned processes because N uptake from the air via leaf assimilation during the green leaf period enriched N in the leaves (Adriaenssens et al. 2012), while the increase in asparagine via protein hydrolysis depleted N in pre-abscission leaves. Resorption, for deciduous species, is mainly in the form of amino acids (Babst and Coleman 2018), when no significant N isotope discrimination was found.
Conclusions
In this study, the NRE and NRP values for beech, larch and cedar were in a similar range compared to values in previous regional studies and to worldwide averages, while for black locust the NRE was substantially lower than previously found in leguminous species. N isotope fractionation was only found during leaf N resorption of beech trees, but only from the green leaf to the pre-abscission period, inferring that it is not due to leaf N resorption but instead is the result of internal N metabolic processes. However, these processes did not induce significant N fractionation in the other species, probably due to variations in the type and amount of free amino acids or the amount of N that is transported as proteins, although this possibility needs further study.
References
Adriaenssens S, Hansen K, Staelens J, Wuyts K, De Schrijver A, Baeten L, Boeckx P, Samson R, Verheyen K (2012) Throughfall deposition and canopy exchange processes along a vertical gradient within the canopy of beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst). Sci Total Environ 420:168–182
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84(4):597–608
Amundson R, Austin AT, Schuur EA, Yoo K, Matzek V, Kendall C, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles 17(1):1031
Aranibar JN, Otter L, Macko SA, Feral CJW, Epstein HE, Dowty PR, Eckardt FD, Shugart HH, Swap RJ (2004) Nitrogen cycling in he soil-plant system along a precipitation gradient in the Kalahari Sands. Glob Change Biol 10(3):359–373
Babst BA, Coleman GD (2018) Seasonal nitrogen cycling in temperate trees: transport and regulatory mechanisms are key missing links. Plant Sci 270:268–277
Chapin FS, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64(2):376–391
Chavez-Vergara B, Merino A, Vázquez-Marrufo G, García-Oliva F (2014) Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 235:133–145
Chavez-Vergara BM, González-Rodríguez A, Etchevers JD, Oyama K, García-Oliva F (2015) Foliar nutrient resorption constrains soil nutrient transformations under two native oak species in a temperate deciduous forest in Mexico. Eur J For Res 134(5):803–817
Codron J, Codron D, Lee-Thorp JA, Sponheimer M, Bond WJ, de Ruiter D, Grant R (2005) Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J Archaeol Sci 32(12):1757–1772
Craine JM, Elmore AJ, Aidar MP, Bustamante M, Dawson TE, Hobbie EA, Nardoto GB (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183(4):980–992
Fotelli MN, Rennenberg H, Gessler A (2002) Effects of drought on the competitive interference of an early successional (Rubus fruticosus) on fagus sylvatica L. seedlings: 15N uptake and partitioning. Responses of amino acids and other N compounds. Plant Biol 4:311–320
Gauthier NP, Soufi B, Walkowicz WE, Pedicord VA, Mavrakis KJ, Macek B, Miller ML (2013) Cell-selective labeling using amino acid precursors for proteomic studies of multicellular environments. Nat Methods 10(8):768
González-Zurdo P, Escudero A, Mediavilla S (2015) N resorption efficiency and proficiency in response to winter cold in three evergreen species. Plant Soil 394(1–2):87–98
Handley LL, Austin AT, Stewart GR, Robinson D, Scrimgeour CM, Raven JA, Schmidt S (1999) The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Funct Plant Biol 26(2):185–199
Hayashi M, Lopez MCL, Nobori Y, Byambasuren M, Boy J (2018) Nitrogen isotope pattern in Mongolian larch stands at the southern Eurasian boreal forest boundary. Isot Environ Health Stud 45(6):608–621
Hobbie EA, Jumpponen A, Trappe J (2005) Foliar and fungal 15N: 14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession—testing analytical models. Oecologia 146(2):258–268
Kahmen A, Wanek W, Buchmann N (2008) Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 156(4):861–870
Killingbeck KT (2004) Nutrient resorption. Plant cell death processes. Academic Press, Cambridge, pp 215–226
Kobayashi H, Tashiro N (2003) Temporal variation in leaf nitrogen content in a Cryptomeria japonica canopy. J Forest Environ (Jpn) 45(2):99–102
Kolb KJ, Evans RD (2002) Implications of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol 156(1):57–64
Lima ALDS, Zanella F, Schiavianto MA, Haddad CRB (2006) N availability and mechanisms of N conservation in deciduous and semideciduous tropical forest legume trees. Acta Bot Bras 20(3):625–632
Lopez CML, Mizota C, Nobori Y, Sasaki T, Yamanaka T (2014) Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia pseudoacacia). J For Res 25(3):585–589
Mae T (2004) Leaf senescence and nitrogen metabolism. Plant cell death processes. Academic Press, Cambridge, pp 157–168
Malagoli M, Dal Canal A, Quaggioti S, Pegoraro P, Botacin A (2000) Differences in nitrate and ammonium uptake between Scots pine and European larch. Plant Soil 221(1):1–3
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D (1996) Leaf 15 N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non-and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 105(1):53–63
Millard P (1994) Measurement of the remobilization of nitrogen for spring leaf growth of trees under field conditions. Tree Physiol 14(7-8-9):1049–1054
Näsholm T (1994) Removal of nitrogen during needle senescence in Scots pine (Pinus sylvestris L.). Oecologia 99(3–4):290–296
Negi GCS, Singh SP (1993) Leaf nitrogen dynamics with particular reference to retranslocation in evergreen and deciduous tree species of Kumaun Himalaya. Can J For Res 23(3):349–357
Oikawa A, Ostuka T, Nakabayashi R, Jikumaru Y, Isuzugawa K, Murayama H, Saito K, Shiratake K (2015) Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS ONE 10(7):e0131408
Özbucak TB, Kutbay HG, Kilic D, Korkmaz H, Bilgin A, Yalcin E, Apaydin Z (2008) Foliar resorption of nutrients in selected sympatric tree species in gallery forest Black Sea region. Pol J Ecol 56(2):227–237
Pardo LH, Templer PH, Goodale CL, Duke S, Groffman PM, Adams MB, Compton J (2006) Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80(2):143–171
Pardo LH, Semaoune P, Schaberg PG, EagarC SM (2013) Patterns in δ 15 N in roots, stems, and leaves of sugar maple and American beech seedlings, saplings, and mature trees. Biogeochemistry 112(1–3):275–291
Santa Regina I, Tarazona T (2001) Nutrient cycling in a natural beech forest and adjacent planted pine in northern Spain. Forestry 74(1):11–28
Schmidt S, Stewart GR (2003) δ15N values of tropical savanna and monsoon forest species reflect root specializations and soil nitrogen status. Oecologia 134(4):569–577
Seidel F, Lopez CML, Guggenberger G, Nobori Y (2017) Impact of low severity fire on soil organic carbon and nitrogen characteristics in Japanese cedar forest, Yamagata Prefecture, Japan. Open J For 7:270–284
Shearer G, Kohl DH (1989) Natural 15N abundance of NH4+, amide N, and total N in various fractions of nodules of peas, soybeans and lupins. Funct Plant Biol 16(4):305–313
Singh A (2014) Nitrogen and phosphorus resorption efficiency in some, native tropical trees planted on a mine spoil in Singrauli Coalfields, India. Int J Environ Bioenergy 9(3):161–170
Singh A (2015) Nitrogen and phosphorus resorption efficiency in some leguminous and non-leguminous tropical tree species planted on coal mine spoil in a tropical dry environment. Ambit J Educ Res Rev 1:1–7
Sun X, Kang H, Chen HY, Björn B, Samuel BF, Liu C (2016) Biogeographic patterns of nutrient resorption from Quercus variabilis Blume leaves across China. Plant Biol 18(3):505–513
Tateno R, Takeda H (2010) Nitrogen uptake and nitrogen use efficiency above and below ground along a topographic gradient of soil nitrogen availability. Oecologia 163(3):793–804
Templer PH, Arthur MA, Lovett GM, Weathers KC (2007) Plant and soil natural abundance δ 15 N: indicators of relative rates of nitrogen cycling in temperate forest ecosystems. Oecologia 153(2):399–406
Ueda MU, Mizumachi E, Tokuchi N (2011) Foliage nitrogen turnover: differences among nitrogen absorbed at different times by Quercus serrata saplings. Annals of Botany 108(1):169–175
van Heerwaarden LM, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101(3):664–669
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82(2):205–220
Vitousek P, Matson PA, Van Cleve K (1989) Nitrogen availability and nitrification during succession: primary, secondary, and old-field seres. Plant Soil 115(2):229–239
Wang L, Ibrom A, Korhonen JFJ, Frumau KF, Wu J, Pihlatie M, Schjoerring JK (2013) Interactions between leaf nitrogen status and longevity in relation to N cycling in three contrasting European forest canopies. Biogeosciences 10:999–1011
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17(1):10–19
Yasumura Y, Onoda Y, Hikosaka K, Hirose T (2005) Nitrogen resorption from leaves under different growth irradiance in three deciduous woody species. Plant Ecol 178(1):29–37
Yoneyama T, Muraoka T, Murakami T, Boonkerd N (1993) Natural abundance of 15N in tropical plants with emphasis on tree legumes. Plant Soil 153(2):295–304
Yuan Z, Chen HY (2009) Global trends in senesced-leaf nitrogen and phosphorus. Glob Ecol Biogeogr 18(5):532–542
Yuan ZY, Li LH, Han XG, Huang JH, Jiang GM, Wan SQ, Zhang WH, Chen QS (2005) Nitrogen resorption from senescing leaves in 28 plant species in a semi-arid region of northern China. J Arid Environ 63(1):191–202
Acknowledgements
We thank technicians Daisuke Arai and Yoshiaki Izuka, of Yamagata University Research Forest and the students of the Watershed Preservation Laboratory, Yamagata University, for their very helpful assistance with fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The online version is available at http://www.springerlink.com
Corresponding editor: Yanbo Hu.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Enta, A., Hayashi, M., Lopez Caceres, M.L. et al. Nitrogen resorption and fractionation during leaf senescence in typical tree species in Japan. J. For. Res. 31, 2053–2062 (2020). https://doi.org/10.1007/s11676-019-01055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-01055-z