Abstract
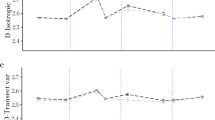
A study was conducted at Msekera Regional Agricultural Research Station in eastern Zambia to (1) describe canopy branching properties of Acacia angustissima, Gliricidia sepium and Leucaena collinsii in short rotation forests, (2) test the existence of self similarity from repeated iteration of a structural unit in tree canopies, (3) examined intra-specific relationships between functional branching characteristics, and (4) determine whether allometric equations for relating aboveground tree biomass to fractal properties could accurately predict aboveground biomass. Measurements of basal diameter (D10) at 10cm aboveground and total height (H), and aboveground biomass of 27 trees were taken, but only nine trees representative of variability of the stand and the three species were processed for functional branching analyses (FBA) of the shoot systems. For each species, fractal properties of three trees, including fractal dimension (Dfract), bifurcation ratios (p) and proportionality ratios (q) of branching points were assessed. The slope of the linear regression of p on proximal diameter was not significantly different (P < 0.01) from zero and hence the assumption that p is independent of scale, a pre-requisite for use of fractal branching rules to describe a fractal tree canopy, was fulfilled at branching orders with link diameters >1.5 cm. The proportionality ration q for branching patterns of all tree species was constant at all scales. The proportion of q values >0.9 (f q ) was 0.8 for all species. Mean fractal dimension (Dfract) values (1.5–1.7) for all species showed that branching patterns had an increasing magnitude of intricacy. Since Dfract values were ≥1.5, branching patterns within species were self similar. Basal diameter (D10), proximal diameter and Dfract described most of variations in aboveground biomass, suggesting that allometric equations for relating aboveground tree biomass to fractal properties could accurately predict aboveground biomass. Thus, assessed Acacia, Gliricidia and Leucaena trees were fractals and their branching properties could be used to describe variability in size and aboveground biomass.
Similar content being viewed by others
References
Allen AP, Pockman WT, Restrepo C, Milne BT. 2008. Allometry, growth and population regulation of the desert shrub Larrea tridentata. Functional Ecology, 22: 197–204.
Betram JEA. 1989. Size-dependent differential scaling in branches: the mechanical design of trees revisited. Trees, 4: 241–253.
Berntson GM. 1996. Fractal geometry scaling and description of plant root. In: A. Eshel and U. Kafkafi (eds), The hidden half. New York: Marcel Dekker, pp. 259–272.
Brown S. 1997. Estimating biomass and biomass change of tropical forests: a primer. FAO Forestry Paper, 134, Rome Italy.
Brown IF, Martineri LA, Thomas WW, Moreira MZ, Ferreira CAC, Victoria RA. 1995. Uncertainty in biomass of Amazonian forests: an example from Rondonia, Brazil. Forest Ecology and Management, 75: 175–189.
Brown JH, Gillooly JH, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology, 85: 1771–1789.
Brown TB, Witschey WRT, Liebovitch LS. 2005. The broken past: Fractals in archaeology. Journal of Archeological Method and Theory, 12(1): 37–78
Camarero JJ, Siso S, Gil-Pelgrin E. 2003. Fractal dimension does not adequately describe the complexity of leaf margins of Quercus species. Real Jardin Botanico de Madrid, 60(1): 63–71
Cannel MGR. 1983. Plant management in agroforestry: manipulation of trees, population densities and mixtures of trees and herbaceous crops. In: P.A. Huxley (ed), Plant Research and Agroforestry. Nairobi, Kenya: ICRAF,, pp. 455–488.
Claesson S, Sahlen K, Lundmark T. 2001. Functions for biomass estimation of young Pinus sylvestris, Picea abies and Betula spp. from stands in Northern Sweden with high stand densities. Scandinavian Journal of forestry, 16: 138–146.
Delitti, WBC, Pausas JG. 2006. Biomass and mineralmass estimates in a “cerrado” ecosystem. Revista Brasileira Botânica, 29(4): 531–40.
Dzerzon H, Sievänen R, Kurth W, Pertunen J, Sloboda B. 2003. Enhanced possibilities for analyzing tree strctures as provided by interface between different modeling systems. Silva Fennica, 37(1): 31–44.
Enquist BJ. 2002. Universal scaling in tree and vascular plant allometry: toward a general quantitative theory linking plant form and functions from cells to ecosystems. Tree Physiology, 22: 1045–1064.
Enquist BJ, Brown JH. West GB. 1998. Allometric scaling of plant energetic and population density. Nature, 395: 163–165.
Fitter AH, Stickland TR. 1992. Fractal characterization of root system architecture. Functional Ecology, 6: 632–635.
Gisiger T. 2001. Scale invariance in biology: coincidence or footprint of a universal mechanism. Biological Review 76: 161–209.
Halley JM, Hartley S, Kallimanis AS, Kunin WE, Lennon JJ, Sgardelis SP. 2004. Uses and abuses of fractal methodology in ecology. Ecology Letters, 7(3): 254–271.
Harrington G. 1979. Estimating aboveground biomass of trees and shrubs in a Eucalyptus populnea F. Mull. Woodland by regression of mass on tree trunk diameter and plant height. Australian Journal of Botany, 27: 135–143.
IPCC. 2006. Guidelines for national greenhouse gas inventories. IGES, ISBN 4-88788-032-4.
IPCC. 2007. Climate Change 2007: The Physical Science Basis. Basic Summary for Policy Makers. Available at http://ipccwg1.ucar.edu/docs/WG1AR4_SPM_PlenaryApproved.pdf.
Jackson NA, Griffiths H, Zeron M. 1995 Aboveground biomass of seedling and semi-mature Sesbania sesban, a multipurpose tree species, estimated using allometric regressions. Agroforestry Systems, 29: 103–112.
Kale M, Sing S, Roy PS, Desothali V, Ghole VS. 2004. Biomass equations of dominant species of dry deciduous forests in Shivupuri district, Madya Pradesh. Current Science, 87(5): 683–687.
Kaonga ML, Coleman L. 2008. Modelling soil organic turnover in improved fallows in eastern Zambia using the RothC model. Forest Ecology and Management, 256(5): 1160–1166.
Kaonga ML, Bayliss-Smith TP. 2009. Carbon pools in tree biomass and the soil in improved fallows in eastern Zambia. Agroforestry Systems, 76: 37–51.
Kaonga ML, Bayliss-Smith TP. 2010. Allometric models for estimation of aboveground carbon stocks in improved fallows in eastern Zambia. Agroforestry Systems, 78: 217–232.
Ketterings QM, Coe R, van Noordiwijk M, Ambagau Y, Palm CA. 2001. Reducing uncertainty in the use of allometric equations for predicting aboveground biomass in mixed secondary forests. Forest Ecology Management, 146: 199–209.
Koziowski J, Konarzewski M. 2004. Is West Brown and Enquist’s model of allometric scaling mathematically correct and biologically relevant. Functional Ecology, 18: 283–289.
Kumar VSK, Tewari VP. 1999. Aboveground biomass tables for Azadrachta indica a. Juss. International Forest Review, 1(2): 109–111.
Litton CM, Kauffman JB. 2008. Allometric models for predicting aboveground biomass in two widespread plants in Hawaii. Biotropica, 40(3): 313–320.
Lott JE, Howard SB, Black CR, Ong CK. (2000) Allometric estimation of above-ground biomass and leaf area in managed Grevillea robusta agroforestry systems. Agroforestry Systems, 49: 1–15.
Makela A, Valentine H. 2006. Crown ratio influences allometric scaling in trees. Ecology, 87(12): 2967–2972.
Masi CEA, Maranville JW. 1998. Evaluation of sorghum root branching using fractals. Journal of Agricultural Sciences, 131: 259–265.
McMahon TA, Kronauer RE. 1976: Tree structures: deducing the principles of mechanical design. Journal of Theoretical Biology, 50: 443–446.
Niklas KJ. 1995. Size-dependent allometry of tree height and trunk diameter. Journal of Botany, 75: 217–227.
Nygren P, Berninger F, Ozier-Lafontaine H, Lecompte F, Ramirez C, Salas E. 1998. Modelling of tree systems in agroforestry. Paper presented at the National Root Seminar, University of Joensuu, 1–2 December 1998.
Ong JE, Gong, WK, Wong CH. 2004. Allometry and partitioning of the mangrove, Rhizophora apiculata. Forest Ecology and Management, 188(1–3): 395–408.
Otieno K, Onim JFM, Bryant MJ, Dzowela BH. 1991. The relation between biomass yield and linear measures of growth in Sesbania sesban in western Kenya. Agroforestry Systems, 13: 131–141.
Ozier-Lafontaine H, Lecompte F, Sillon JN. 1999. Fractal analysis of root architecture of Gliricidia for spatial prediction of root branching, size and mass: model development and evaluation in agroforestry. Plant Soil, 209: 167–180.
Price CA, Enquist B. 2007. Scaling mass and morphology in leaves:an extension of the WBE model. Ecology, 88(5): 1132–1141.
Richardson AD, zu Dohna H. 2003. Predicting root biomass from branching patternsof Douglas-fir rooting systems. Oikos, 100: 96–104.
Saatchi SS, Houghton A, Dos Santos Alvala RC, Soare JV, Yu Y. 2007. Distribution of aboveground biomass in the Amazon. Global Change Biology, 13: 816–837.
Sağlan B, Kűcűki O, Bilgili E, Durmaz D, Basal I. 2008. Estimating fuel biomass of some shrub species (Maquis) in Turkey. Turkish Journal of Agriculture, 32: 349–356.
Saint-André L, M’bou Mabiala A, Mouvondy W, Jourdan C, Roupsard A, Deleporte P, Hamel O, Nouvellon Y. 2005. Age-related equations for above- and below-aground biomass of Eucalyptus hybrid in Congo. Forest Ecology and Management, 205: 199–214.
Smith DM. 2001 Estimation of tree root lengths using fractal branching rules: a comparison with soil coring for Grevillea robusta. Plant Soil, 229: 295–301.
Snowdon P. 1991 A ratio estimator for bias correction in logarithmic regressions. Canadian Journal of Forest Research, 21: 720–724.
Spek LY, Van Noordwijk M. 1994 Proximal root diameter as a predictor of total root size for fractal models. II. Numerical model. Plant soil, 164: 119–127.
Sugihala G, May RM. 1990 Applications of fractals in ecology. Tree, 5(3):79–86.
Tucote DL, Pelletier JD, Newman WI. 1998. Networks with side branching in biology. Journal of Theoretical Biology, 193: 577–592.
Van TK, Rayachhetry MB, Centre D. 2000. Estimating aboveground biomass of Melaleuca quinquenenervia in Florida, USA. Journal of Aquatic Plant Management, 38, 62–67.
Van Noordwijk M, Mulia R. 2002. Functional branch analysis as a tool for fractal scaling above- and belowground trees for their additive and nonadditive properties. Ecological Modelling, 149: 41–51.
Van Noordwijk M, Spek LY, De Willigen P. 1994. Proximal root diameters as predictors of total root size for fractal branching models. I. Theory. Plant Soil, 164: 107–118.
West GB, Brown JH, Enquist BJ. 1999: A general model for the structure and allometry plant vascular systems. Nature, 400: 664–667.
William N. 1997. Fractal geometry gets measure of life scales. Science 276: 34.
Author information
Authors and Affiliations
Corresponding author
Additional information
Founadition project: The study was funded by the Gates Cambridge Trust at Cambridge University.
Rights and permissions
About this article
Cite this article
Kaonga, M.L. Fractal analysis of canopy architectures of Acacia angustissima, Gliricidia sepium, and Leucaena collinsii for estimation of aboveground biomass in a short rotation forest in eastern Zambia. Journal of Forestry Research 23, 1–12 (2012). https://doi.org/10.1007/s11676-012-0227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-012-0227-7