Abstract
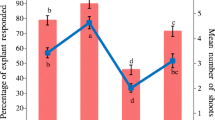
Different nutrient media can affect in vitro culturing protocols, and experimentation under varied growth conditions is valuable in plants where in vitro methods are in preliminary stages. We carried out the first in vitro propagation studies for the endangered species Caragana fruticosa (Fabaceae). We evaluated various nutrient media for their impact on shoot elongation and axillary bud proliferation using different concentrations of 6-benzylaminopurine (BA) and α-naphthaleneacetic acid (NAA). Shoot elongation was evaluated based on adventitious shoot primary culture and subculture regeneration from Caragana seedlings. Our goal was to improve both micropropagation and regeneration in C. fruticosa. MS nutrient media was superior to 1/2MS macronutrients, DKW, QL, and WPM for shoot elongation and axillary shoot proliferation. Shoots grown on 1/2MS and WPM exhibited some chlorosis, and shoots on QL produced larger leavers than plants growing on normal medium. The shoot proliferation coefficient on MS media supplemented with 2.22 μM BA and 0.44 μM BA + 2.69 μM NAA was significantly higher than that with other treatments in the primary culture. Shoots on 2.22 μM BA showed a higher proliferation coefficient (3.17) than others in the subculture. Shoots were rooted on 1/2MS medium with the addition of different concentrations of NAA. The optimal concentration for rooting was 0.27 μM NAA (74%). Roots exhibited many stout and long root hairs. Survivl of established plantlets was 82% at 30 days after transfer to soil. Plants established in the green house showed normal growth and displayed no apparent morphological differences compared to stock plants.
Similar content being viewed by others
Abbreviations
- 1/2MS:
-
Half strength of MS macronutrients
- BA:
-
6-Benzylaminopurine
- DKW:
-
Driver-Kuniyuki walnut medium
- MS:
-
Murashige and Skoog medium
- NAA:
-
α-Naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- QL:
-
Quoirin and Lepoivr’s medium
- WPM:
-
Woody Plant Medium
References
Andreu P, Marín JA. 2005. In vitro culture establishment and multiplication of the Prunus rootstock ‘Adesoto 101’ (P. insititia L.) as affected by the type of propagation of the donor plant and by the culture medium composition. Scientia Horticulturae, 106(2): 258–267.
Bell RL, Srinivasan C, Lomberk D. 2009. Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cellular and Developmental Biology, 45: 708–714.
Cheng KJ, Ma DY, Yang GX, Hu CQ. 2008. A new tetrastilbene from Caragana sinica. Chinese Chemical Letters, 19(6): 711–715.
Driver JA, Kuniyuki H. 1984. In vitro propagation of Paradox walnut rootstock. HortScience, 19: 507–509.
Gao Mei, Li Tianran. 1990. Relationship between organogenesis and endogenous hormone contents of some wild Legume plants from genus Caragana Fabr. cultured in vitro. Journal of Inner Mongolia University (Acta Scientiarum Naturalium Universitatis NeiMongol), 21(3): 427–437. (in Chinese)
Guo Liangang, Ma Runlan, He Xiaoyong. 2007. Immature embryos cultivation and propagation of Caragana korshinskii. Inner Mongolia Agricultural Science and Technology, (5): 46–47. (in Chinese)
He S, Liu C, Saxena PK. 2007. Plant regeneration of an endangered medicinal plant Hydrastis canadensis L.. Scientia Horticultura,113(1): 82–86.
Hu Namei, Han Suying, Liang Guolu, Qi Liwang. 2009. Inducing Regeneration of Caragana intermedia by TDZ. Northern Horticulture, (8): 204–205. (in Chinese)
Huo Xiaolan, Yan Min, Zhang Qiang, Meng Quanye. 2009. Effects of different plant hormone concentrations on tissue culture Stem of Caragana microphylla. Chinese Agricultural Science Bulletin, 25(10): 148–150. (in Chinese)
Huo Y, Guo C, Zhang QY, Chen WS, Zheng HC, Rahman K, Qin LP. 2007. Antinociceptive activity and chemical composition of constituents from Caragana microphylla seeds. Phytomedicine, 14(2/3): 143–146.
Ji Meng, Yang Wenbin, Liang Hairong, Qi Liwang, Wang Yangdong. 2006. Preliminary Research on Cuttage by Epicormic Branch of Caragana intermedia Kuang et H.C. Fu. Inner Mongolia Forestry Science and Technology, (1): 5–9. (in Chinese)
Jia Li, Qu Shizeng. 2001. The study progress on the genus Caragana Fabr.. Bulletin of Botanical Research, 21(4): 515–518. (in Chinese)
Liu Shene. 1955. Illustrated manual of woody plants of Northeast China. Beijing, Science Publishing, pp.335. (in Chinese)
Lloyd G, McCown B. 1980. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Plant Prop Soc CombProc, 30: 421–427.
Lu MC. 2005. Micropropagation of Vitis thunbergii Seib. Et Zucc., a medici-nal herb, through high-frequency shoot tip culture. Scientia Horticulturae, 107(1): 64–69.
Lu YL, Chen WF, Wang ET, Guan SH, Yan XR, Chen WX. 2009. Genetic diversity and biogeography of rhizobia associated with Caragana species in three ecological regions of China. Systematic and Applied Microbiology, 32(5): 351–361.
Ma CC, Gao YB, Guo HY, Wang JL, Wu JB, Xu JS. 2008. Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. Journal of Arid Environments, 72(3): 247–254.
Ma Jingping. 2007. Technique on seedling and forestation of Caragana opulens. Science and Technology of Qinghai Agriculture and Forestry, 2: 49–50. (in Chinese)
Mhatre M, Salunkhe CK, Rao PS. 2000. Micropropagation of Vitis vinifera L.: towards an improved protocol. Scientia Horticulturae, 84(3/4): 357–363.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant, 15(3): 473–497.
Niu Xiwu, Yang Huizhen, Zhan Haixian, Ren Zhiqiang, Chang Zhijian, Yang Lili. 2004. Tissue culture and rapid propagation of peashrub. Acta Bot Boreal Occident Sin, 24(8): 1502–1505. (in Chinese)
Offord CA, Tyler JL. 2009. In vitro propagation of Pimelea spicata R.Br (Thymeleaceae), an endangered species of the Sydney region, Australia. Plant Cell Tissue Organ Cult, 98: 19–23.
Papiya M, Nafisa HSC, Misra VSR. 2010. In vitro propagation of a grape rootstock, deGrasset (Vitis champinii Planch): Effects of medium compositions and plant growth regulators. Scientia Horticulturae, 126: 13–19.
Peddaboina V, Thamidala C, Karampuri S. 2006. In vitro multiplication and plant regeneration in four Capsicum species using thidiazuron. Scientia Horticulturae, 107: 117–122.
Qin YM, Wang D, Chi FC. 1993. Rare and endangered plants in Heilongjiang proviance. Harbin: Northeast Forestry University Publishing, 115–117.
Quoirin M, Lepoivre P. 1977. Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Hortic, 78: 437–442.
Sanczir C. 2003. New taxa of the genus Caragana (Fabaceae) from the Middle Asia. Bot Zhurn, 88: 106–108
Song Junshuang, Wang Zan, Sun Guizhi, Gao Hongwen. 2007. Study on the tissue culture of Horqin peashrub. Acta agrestia sinica, 15(1): 66–69. (in Chinese)
Toit JT, Kock R, Deutsch J. 2009. Wild rangelands: Conserving wildlife while maintaining livestock in semi-arid ecosystems. Blackwell Publishing Ltd, 10: 291–311.
Wei Yanbo, Shen Xiaohui, Lin Yumei, Ren Jun, Yang Zhiyin, Zhang Lin, Song Liwen, Song Fuqiang. 2000. Study on declination of seed vitality of Caragana microphylla. Jilin Forestry Science and Technology, 29: 12–14. (in Chinese)
Xiang T, Uno T, Ogino F, Ai C, Duo J, Sankawa U. 2005. Antioxidant constituents of Caragana Tibetica. Chem Pharm Bull, 53(9): 1204–1206.
Yan Xingfu. 2007. Propagulum of Caragana spp. and their roles in restoration of deserticolo. Inner Mongolia Forestry Science and Technology, 33: 23–27. (in Chinese)
Yang Chengli, Fan Xiaojuan, Yang Junfang, Li Dali. 2007. Tissue culture and rapid propagation of Caragana sinica (Buchoz) Rehd. Plant physiology communications, 43(6): 1130. (in Chinese)
Yong ZS, Zhao HL. 2003. Soil properties and plant species in an age sequence of Caragana microphylla plantations in the Horqin Sandy Land, north China. Ecological Engineering, 20(3): 223–235.
Zhang Errong, Chen Fen. 1990. The rapid propagation on tissue culture of Caragana arborgce. Journal of Northeast Agricultural College, 21(1): 94–97. (in Chinese)
Zhang Qiang, Guo Sujuan, Zhai Mingpu. 2005. Technology of tissue culture in Caragana microphylla. Journal of Fujian College of Forestry, 25(3): 256–259. (in Chinese)
Zhang Z, Wang SP, Nyrene P, Jiang GM. 2006. Morphological and reproductive response of Caragana microphylla to different stocking rates. Journal of Arid Environments, 67: 671–677.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation project: This research was supported by the Key Technologies R&D Program of China during 2006–2010 (2006BAD03A04) and the Fundamental Research Funds for the Central Universities (DL10BA04).
The online version is available at http://www.springerlink.com
Rights and permissions
About this article
Cite this article
Zhai, Xj., Yang, L. & Shen, Hl. Shoot multiplication and plant regeneration in Caragana fruticosa (Pall.) Besser. Journal of Forestry Research 22, 561–567 (2011). https://doi.org/10.1007/s11676-011-0199-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-011-0199-z