Abstract
Objective
The Notch signaling pathway plays an important role in the stem cell signaling network and contributes to tumorigenesis. However, the functions of Notch signaling in ovarian cancer stem cells (OCSCs) are not well understood. We aimed to investigate the effects of Notch blockade on self-renewal and stemness maintenance of OCSCs.
Methods
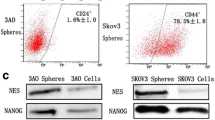
Ovarian cancer stem-like cells were enriched from ovarian cancer cell lines in serum-free medium. A γ-secretase inhibitor, (DAPT), was used to block Notch signaling. MTT assays were performed to assess self-renewal and proliferation inhibition, flow cytometry was performed to analyze cell surface marker and immunofluorescence, Western Blot and Real-time RT-PCR assays were performed to detect Oct4 and Sox2 protein and mRNA expression of the Ovarian cancer stem-like cells treated with DAPT.
Results
Notch blockade markedly inhibits self-renewal and proliferation of ovarian cancer stem-like cells, significantly downregulates the expression of OCSCs-specific surface markers, and reduces protein and mRNA expression of Oct4 and Sox2 in OCSC-like cells.
Conclusion
Our results suggest that Notch signaling is not only critical for the self-renewal and proliferation of OCSCs, but also for the stemness maintenance of OCSCs. The γ-secretase inhibitor is a promising treatment targeting OCSCs.
Similar content being viewed by others
References
Pecorelli S, Favalli G, Zigliani L, et al. Cancer in women. Int J Gynaecol Obstet 2003; 82: 369–379.
Dick JE. Stem cell concepts renew cancer research. Blood 2008; 112: 4793–4807.
Clarke MF, Dick JE, Dirks PB, et al. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res 2006; 66: 9339–9344.
Zhang S, Balch C, Chan MW, et al: Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res 2008; 68: 4311–4320.
Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol 2007; 18: 460–466.
Bapat SA, Mali AM, Koppikar CB, et al. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 2005; 65: 3025–3029.
Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol 2004; 93: 170–181.
Curley MD, Therrien VA, Cummings CL, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells 2009; 27: 2875–2883.
Wani AA, Sharma N, Shouche YS, et al. Nuclear-mitochondrial genomic profiling reveals a pattern of evolution in epithelial ovarian tumor stem cells. Oncogene 2006; 25: 6336–6344.
Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA 2006; 103: 11154–11159.
Mimeault M, Hauke R, Mehta PP, et al. Recent advances in cancer stem/progenitor celll research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 2007; 11: 981–1011.
Leong KG, Karsan A. Recent insight into the role of Notch signaling in tumorigenesis. Blood 2006; 107: 2223–2233.
AI-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene 2004; 23: 7274–7282.
Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science 1999; 284: 770–776.
Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature 2006; 442: 823–826.
Li JL, Harris AL. Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell 2005; 8: 1–3.
Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res 2004; 64: 7794–7800.
Sjölund J, Johansson M, Manna S, et al. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest 2008; 118: 217–228.
Kimura K, Satoh K, Kanno A, et al. Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice. Cancer Sci 2007; 98: 155–162.
Rappa G, Mercapide J, Anzanello F, et al. Growth of cancer cell lines under stem cell-like conditions has the potential to unveil therapeutic targets. Exp Cell Res 2008; 314: 2110–2122.
Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res 2003; 993: 18–29.
Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–1270.
Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res 2009; 19: 683–697.
Reya T, Morrison SJ, Clarke MF, et al. Stem cells,cancer,and cancer stem cells. Nature 2001; 414: 105–111.
Bray SJ. Notch signaling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7: 678–689.
Katoh M, Katoh M. Notch signaling in gastrointestinal tract. Int J Onco 2007; 30: 247–251.
Shih IeM, Wang TL. Notch Signaling, gamma-Secretase Inhibitors, and Cancer Therapy. Cancer Res 2007; 67: 1879–1882.
Raspollini MR, Amunni G, Villanucci A, et al. c-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann Oncol 2004; 15: 594–597.
Saegusa M, Machida D, Hashimura M, et al. CD44 expression in benign, premalignant, and malignant ovarian neoplasms: relation to tumour development and progression. J Pathol 1999; 189: 326–337.
Shmelkov S, Clair RSt, Lyden D, et al. AC133/CD133/Prominin-1. Int J Biochem Cell Biol 2005; 37: 715–719.
Mimeault M, Hauke R, Mehta PP, et al. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 2007; 11: 981–1011.
Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene 2009; 28: 209–218.
Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 2008; 26: 1269–1275.
Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40: 499–507.
Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis 2007; 25: 217–229.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from the Heilongjang Province Science and Technology Commission of China (No. GB07C32304)
Rights and permissions
About this article
Cite this article
Jiang, Ly., Zhang, Xl., Du, P. et al. γ-Secretase inhibitor, DAPT inhibits self-renewal and stemness maintenance of ovarian cancer stem-like cells in vitro . Chin. J. Cancer Res. 23, 140–146 (2011). https://doi.org/10.1007/s11670-011-0140-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11670-011-0140-1