Abstract
The partial and integral enthalpies of mixing of liquid ternary Ni-Sn-Zn alloys were determined. The system was investigated along two sections x Ni/x Sn ≈ 1:9, x Ni/x Sn ≈ 1:6 at 1073 K and along two sections x Sn/x Zn ≈ 9:1, x Sn/x Zn ≈ 4:1 at 873 K. The integral enthalpy of mixing at each temperature is described using the Redlich-Kister-Muggianu model for substitutional ternary solutions. In addition, the experimental results were compared with data calculated according to the Toop extrapolation model. The minimum integral enthalpy of approx. −20000 J mol-1 corresponds to the minimum in the constituent binary Ni-Sn system, the maximum of approx. 3000 J mol-1 is equal to the maximum in the binary Sn-Zn system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low temperature soldering is one of the key technologies for the production of electronics devices. Recently, several types of new lead-free Sn-based solders have attracted the attention of the electronics industry.[1-4] Eutectic or near eutectic Sn-Zn alloys, which have been recognized as possible solder candidates due to their low melting temperatures and low costs, are among them.
The melting point of the eutectic Sn85.1Zn14.9 solder is 472 K, which is close to that of the conventional Sn-Pb eutectic alloy (456 K) but lower than those of other Sn-based eutectic alloys that are already used in soldering, i.e. Sn-Cu (500 K), Sn-Ag (494 K) or Sn-Ag-Cu (490 K). While the Sn-Zn eutectic alloy has excellent properties as a low temperature solder, it has also some drawbacks. Damage by heat exposure and corrosion in humidity, inferior wettability, easy oxidation and micro-void formation have been encountered to limit the practical use of this solder.[5]
It is known that the poor oxidation resistance of the Sn-Zn eutectic alloy is due to zinc oxidation which occurs both in the primary crystallization of (Zn) and the eutectic phase. If the amount of the (Zn) phase in the Sn-Zn eutectic alloy can be reduced or fixed by formation of intermetallic compounds, it is expected that the oxidation resistance can be improved.[6] Therefore, much research was focused on the addition of alloying elements, such as Cu, Ni, Ag, Sb, or Bi,. Among them, Ni has been considered as a suitable alloying element in lead-free solders due to the formation of stable Ni-Zn binary phases as well as by improving the wettability.[7] Furthermore, the addition of Ni effectively enhances the formation of additional ternary intermetallic compounds which can improve the mechanical properties.[8]
The development of lead-free solders requires a clear and thorough understanding of their structural and thermodynamic properties. The increasing influence of computational modelling in all technological processes generates an increased demand for accurate thermodynamic information for the materials systems involved, which are used as fundamental inputs for any model. The solidification process of a liquid alloy has a profound impact on the structure and properties of the solid material. Therefore, knowledge of the basic properties of the molten alloys prior to solidification becomes very important for the development of materials with predetermined characteristics.
In this work the enthalpy of mixing of Sn-based liquid Ni-Sn-Zn alloys was investigated at 873 and 1073 K. The data obtained are useful for modelling of interatomic interactions of the components as well as for a thermodynamic assessment of the Ni-Sn-Zn system. The experimental data were fitted on the basis of an extended Redlich-Kister-Muggianu model[9] and compared to data calculated according to the Toop extrapolation model.[10]
Literature Survey
The Sn-Zn Binary System
Thermodynamic properties of liquid Sn-Zn alloys have been investigated repeatedly.[11-20] The authors used different methods to determine the enthalpy of mixing: calorimetric investigations were carried out in Ref 11-15, the authors of Ref 16-19 used the emf method, and quantitative thermal analysis was used in Ref 20. An endothermic behavior of ΔMix H has been revealed over the whole concentration region with a maximum point at about 65 at.% Zn; only Kleppa reported a temperature dependence of the integral enthalpy of mixing.[11] A critical review of the experimental enthalpy of mixing data was published by Lee.[21]
The Ni-Sn Binary System
The enthalpy of mixing of liquid Ni-Sn alloys was investigated experimentally in Ref 22-24. According to Haddad et al.,[22] the enthalpy of mixing does not depend on temperature between 867 and 1579 K while such a dependence was reported in the temperature range 1660-1775 K by Lück et al.[23]
A strong temperature dependence of the limiting partial enthalpy of mixing for Ni in Sn was observed by Flandorfer et al.[24] The authors concluded also a certain temperature dependence of the integral enthalpy of mixing in the liquid state near the liquidus curve. Several thermodynamic assessments including phase diagram calculations, based on experimental data, were carried out in the past.[25-27]
The Ni-Zn Binary System
Experimental data on the thermodynamic properties of liquid Ni-Zn alloys are scarce in the literature[28,29]; all investigations point to an exothermic mixing behavior. The minimum point, shifted to the Zn-reach side from the equiatomic concentration, can be explained by the existence of short-range order in the liquid corresponding to the rather stable γ-phase in the solid state. All thermodynamic optimizations were based on the same experimental data.[30-33]
The Ni-Sn-Zn Ternary System
No calorimetric data for liquid Ni-Sn-Zn alloys have been reported up to now. However, Gandova et al.[36] attempted an extrapolation from binary thermodynamic data to obtain Gibbs energy values for ternary liquid alloys, using different geometrical models as well as the CALculation of PHAse Diagrams (CALPHAD) method. Various groups of authors reported partial ternary phase diagrams, especially isothermal sections at different temperatures.[34,35,37,41,42]
Experimental Procedure
A Calvet-type microcalorimeter HTMC-1000 (Setaram, Lyon, France), equipped with an automatic drop device for up to 30 drops, was used for the enthalpy of mixing measurements.[38] Control and data evaluation was done with Lab View and HiQ. All measurements were performed under Ar flow (approx. 30 cm3/min) in graphite crucibles. The microcalorimeter was calibrated at the end of each measurement series by five additions (approx. 40 mg each) of standard α-Al2O3 supplied by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). The interval between individual drops was 40 min, the acquisition interval of the heat flow was about 0.5 s. Two thermopiles with more than 200 thermocouples of Pt/Pt-10Rh were used for the determination of the sample temperature (T M) in the furnace and of the corresponding heat effect for each drop. The measured enthalpy ∆H Signal (integrated heat flow at constant pressure) is given by
where n i is the number of moles of the added sample, H m denotes molar enthalpies, and T D is the drop temperature (room temperature). The molar enthalpy difference \((H_{{{\text{m,}}i ,T_{\text{M}} }} - H_{{{\text{m,}}i ,T_{\text{D}} }} )\) was calculated using the SGTE data for pure elements.[39] Because of the rather small masses added, the partial enthalpies can be given directly as
The integral enthalpy of mixing was calculated by summarizing the respective reaction enthalpies and dividing by the total molar amount of substance. The respective binary starting value for each section in the ternary system was calculated from the binary literature data[24,32,40] using the interaction parameters listed in Table 3.
The enthalpy of mixing for ternary liquid Ni-Sn-Zn alloys was determined along two sections at 873 K where pure Ni was dropped into liquid Sn1−x Zn x alloys (x = 0.09 and 0.18) as well as along two section at 1073 K where pieces of pure Zn were dropped into liquid Ni x Sn1−x alloys (x = 0.10 and 0.15) (Fig. 1). A lower temperature of 873 K was chosen for sections C and D to avoid excessive Zn losses by evaporation. For sections A and B a higher temperature of 1073 K was chosen in order to cover a larger liquid range.
Random errors as well as systematic errors of calorimetry depend on different factors, such as construction of the calorimeter, calibration procedure, signal integration or “chemical errors”, e.g. incomplete reactions or impurities. Considering many calibration measurements done by dropping NIST standard sapphire, the standard deviation can be estimated to be less than ±1%. The systematic errors are mainly caused by parasitic heat flows, base line problems at signal integration and dropping and mixing problems. One can estimate that the random error of the measured enthalpy is about ±150 J.
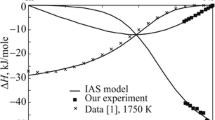
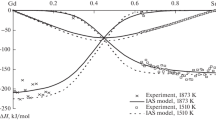
All experimental details, i.e. starting amounts, added amounts and resulting heat effects as well as the obtained enthalpy of mixing values are collected in Tables 1 and 2. Figure 2, 3, 4, and 5 show the changes of ∆Mix H versus concentration.
Results and Discussion
Experimental Results
According to the phase equilibria at 1073 K,[35] the experimental temperature for (Ni-Sn) + Zn alloys along the sections A (x Ni/x Sn ≈ 1:9) and B (x Ni/x Sn ≈ 1:6) was high enough to obtain completely liquid alloys over the entire investigated concentration range; see dashed-points line in Fig. 1. In contrary, the clear kinks in the enthalpy curves for (Sn-Zn) + Ni alloys along the cross sections C (x Sn/x Zn = 9:1) and D (x Sn/x Zn ≈ 4:1) shown in Fig. 4 and 5 indicate formation of a solid phase and denote the liquidus limit at 873 K. The corresponding points are in reasonable agreement with the estimated liquidus line at 873 K given by Yuan et al.[37] which is shown as a dashed line in Fig. 1. Accordingly, the values within the italicized values in Table 2 are for alloys beyond the liquidus limit.
The obtained enthalpies of mixing are exothermic along all sections, indicating the preferred interactions between unlike kinds of atoms. It should be noted that the enthalpy of mixing data for both (Sn-Zn) + Ni alloys are practically identical in the concentration range after formation of a solid phase. This may be explained by formation of the same phase in both cases.
Ternary Modeling
The interaction parameters of the binary systems were taken directly from the literature[24,32,40] and are listed in Table 3. The enthalpy of mixing for the ternary system was treated by a least-squares fit using the following Redlich-Kister-Muggianu polynomial,[9] which takes into account additional ternary interactions:
where i, j, k are equal to 1, 2, 3 for the elements Ni, Sn and Zn respectively; ν L i:j (ν = 0, 1, 2,…) are the interaction parameters of the three binary systems; ν M i:j:k (ν = 0, 1, 2) are three ternary interaction parameters; x i , x j , x k are the corresponding mole fractions. The enthalpy of mixing is temperature independent for the two binary systems Ni-Zn and Sn-Zn, and it shows small temperature dependence for the Ni-Sn system. Therefore, any possible temperature dependence of ΔMix H in the ternary Ni-Sn-Zn system was neglected in the present evaluation. The parameters ν M i:j:k , obtained from the experimental enthalpy of mixing data, represent the additional contribution due to ternary interactions (Table 3). The difference between experimental and calculated enthalpy of mixing data is not more than ±250 J mol-1 which is within the limits of the experimental errors. This can be seen from Fig. 2, 3, 4, and 5 where full lines refer to calculated values with ternary interaction, dashed lines to those without.
As an alternative, the so-called Toop model[10] was used to calculate the ternary enthalpy values. This model uses an asymmetric extrapolation to predict ternary thermodynamic quantities based on binary data. The corresponding equation is:
where ∆Mix H i,j , ∆Mix H i,k , and ∆Mix H j,k , are the enthalpies of mixing for liquid Ni-Sn, Ni-Zn and Sn-Zn alloys, respectively. The enthalpies of mixing values of binary sub-systems were calculated by a Redlich-Kister polynomial based on interaction parameters from the literature given in Table 3.
A comparison of the experimental enthalpy of mixing with the calculated data along all investigated cross sections is shown in Fig. 2, 3, 4, and 5. It can be seen that the calculated curves based on the Toop model are in good agreement with our fitting without ternary interaction terms and differ from the experimental data by less than 400 J mol-1 except for the section x Ni/x Sn ≈ 1:9 where the deviation is higher. This comparatively small improvement of the fits adding ternary interaction terms, however, is not a proof for the existence of real ternary interaction in the liquid phase. Both, the Muggianu- and the Toop-model for the extrapolation of binary enthalpy data into the ternary are of limiting significance. Thus the ternary terms could also compensate shortcomings of the binary extrapolations models
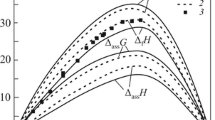
Finally, an iso-enthalpy plot is presented in Fig. 6. The values are exothermic in most of the ternary composition range, except close to the binary Sn-Zn system. The minimum values are actually in the binary Ni-Sn system. All data beyond the liquidus limit are considered as enthalpy of mixing of the metastable liquid.
Conclusions
Enthalpies of mixing in the liquid Ni-Sn-Zn system were measured along four sections using a high temperature Calvet microcalorimeter. For two sections x Ni/x Sn ≈ 1:9, x Ni/x Sn ≈ 1:6 were measured at 1073 K, for the other two sections, i.e. x Sn/x Zn ≈ 9:1, x Sn/x Zn ≈ 4:1, experiments were performed at 873 K. A comparison of experimental and calculated enthalpy of mixing values based on Redlich-Kister-Muggianu data fits and on the Toop extrapolation model shows good agreement.
Based on the experimental data three ternary interaction parameters ν M i:j:k were obtained according to the Redlich-Kister-Muggianu polynomial. These data could be used in a standard CALPHAD procedure for the assessment of the equilibrium phase diagram.
References
K.J. Puttlitz and K.A. Stalter, Handbook of Lead-Free Solder Technology for Microelectronic Assemblies, Marcel Dekker Inc., New York, 2004
K.S. Kim, J.M. Yang, C.H. Yu, I.O. Jung, and H.H. Kim, Analysis on Interfacial Reactions Between Sn-Zn Solders and the Au/Ni Electrolytic-Plated Cu Pad, J. Alloys Compd., 2004, 379, p 314-318
I. Shohji, T. Nakamura, F. Mori, and S. Fujiuchi, Interface Reaction and Mechanical Properties of Lead-free Sn-Zn Alloy/Cu Joints, Mater. Trans., 2002, 43, p 1797-1801
K. Suganuma and K.S. Kim, Sn-Zn Low Temperature Solder, J. Mater. Sci. Mater. Electron., 2007, 18, p 121-127
X.Q. Wei, H.Z. Huang, L. Zhou, M. Zhang, and X.D. Liu, On the Advantages of Using a Hypoeutectic Sn-Zn as Lead-Free Solder Material, Mater. Lett., 2007, 61, p 655-658
J.X. Jiang, J.E. Lee, K.S. Kim, and K. Suganuma, Oxidation Behavior of Sn-Zn Solders Under High-Temperature and High-Humidity Conditions, J. Alloys Compd., 2008, 462, p 244-251
W.J. Zhu, H.S. Liu, J.S. Wang, G. Ma, and Z.P. Jin, Interfacial Reactions Between Sn-Zn Alloys and Ni Substrates, J. Electron. Mater., 2010, 39, p 209-214
A.K. Gain, Y.C. Chan, and W.K.C. Yung, Effect of Nano Ni Additions on the Structure and Properties of Sn-9Zn and Sn-Zn-3Bi Solders in Au/Ni/Cu Ball Grid Array Packages, Mater. Sci. Eng. B, 2009, 162, p 92-98
Y.M. Muggianu, M. Gambino, and J.P. Bros, Enthalpies of Formation of Liquid Alloys Bismuth-Gallium-Tin at 723 K—Choice of an Analytical Representation of Integral and Partial Thermodynamic Functions of Mixing for This Ternary-System, J. Chim. Phys., 1975, 72, p 83-88
G.W. Toop, Predicting Ternary Activities Using Binary Data, Trans. Met. Soc. AIME, 1965, 233, p 850-855
O.J. Kleppa, A Thermodynamic Study of Liquid Metallic Solutions. 6. Calorimetric Investigations of the Systems Bismuth-Lead, Cadmium-Lead, Cadmium-Tin and Tin-Zinc, J. Phys. Chem., 1955, 59, p 354-361
W. Oelsen, Zur Kalorimetrie Und Thermodynamik Der Zinn-Zink-Legierungen, Z. Metallkd., 1957, 48, p 1-8, in German
E. Schürmann and H. Träger, Die Empfindlichkeit und die Wiederholbarkeit von Messungen mit dem Kleinkalorimeter (The Sensitivity and Reproducibility of Measurements with Microcalorimeter), Arch. Eisenhüttenwes., 1961, 32, p 397-408
Z. Moser and R.S. K. Rzyman, Calorimetric Studies on Zn-Sn Liquid Solutions, Bull. Pol. Acad. Sci. Tech. Sci., 1987, 35, p 461-464
M. Genot and R. Hagege, Etude Thermodynamique Du Systeme Etainzinc, Compt. Rend. Hebd. Acad. Sci., 1960, 251, p 2901-2903, in French
W. Ptak, Thermodynamics of Liquid Zinc-Tin Solutions, Archiwum Hutnictwa, 1960, 5, p 649-650
K. Sano, K. Okajima, and S. Tatsuo, A Thermodynamic Study of the Liquid Ternary System Zinc-Cadmium and Zinc-Tin. III. The System Zinc-Cadmium-Tin, Mem. Fac. Eng. Nagoya Univ., 1953, 5, p 299-305
M. Fiorani and V. Valenti, Ricerche Termodinamiche su Sistemi Metallici - Nota III. Sistema Liquido Zonco-Stango, Gazz. Chim. Ital., 1955, 85, p 607-615, in Italian
E. Scheil and E.D. Muller, Dampfdruckmessungen an Flussigen Zink-Zinn-Legierungen, Z. Metallkd., 1962, 53, p 389-395, in German
K. Itagaki and A. Yazawa, Measurements of Thermo-Dynamic Quantities for Tin-Zinc and Indium-Antimony Alloys by Quantitative Thermal Analysis, J. Jpn. Inst. Met., 1975, 39, p 880-887
B.J. Lee, Thermodynamic Assessments of the Sn-Zn and In-Zn Binary Systems, CALPHAD, 1996, 20, p 471-480
R. Haddad, M. Gaune-Escard, J.P. Bros, A. Ranninger-Havlicek, E. Hayer, and K.L. Komarek, Thermodynamics of Nickel-Tin Liquid Alloys, J. Alloys Compd., 1997, 247, p 82-92
R. Luck, J. Tomiska, and B. Predel, Calorimetric Determination of the Enthalpy of Mixing of Liquid Nickel-Tin Alloys as a Function of Temperature, Z. Metallkd., 1988, 79, p 345-349, in German
H. Flandorfer, C. Luef, and U. Saeed, On the Temperature Dependence of the Enthalpies of Mixing in Liquid Binary (Ag, Cu, Ni)-Sn Alloys, J. Non-Cryst. Solids, 2008, 354, p 2953-2972
P. Nash, H. Choo, and R.B. Schwarz, Thermodynamic Calculation of Phase Equilibria in the Ti-Co and Ni-Sn Systems, J. Mater. Sci., 1998, 33, p 4929-4936
G. Ghosh, Thermodynamic Modeling of the Nickel-Lead-Tin System, Metall. Mater. Trans. A, 1999, 30, p 1481-1494
H.S. Liu, J. Wang, and Z.P. Jin, Thermodynamic Optimization of the Ni-Sn Binary System, CALPHAD, 2004, 28, p 363-370
H. Hagiwara, S. Sugino, and K. Yamaguchi, Activity of Zinc in Liquid Nickel-Zinc Alloys, Bull. Univ. Osaka Prefect. Ser. A, 1977, 26, p 81-86
T.G. Chart, J.K. Critchle, and R. Williams, Thermodynamic Data for Nickel-Zinc Alloys, J. Inst. Met., 1968, 96, p 224
G.P. Vassilev, T. Gomez-Acebo, and J.C. Tedenac, Thermodynamic Optimization of the Ni-Zn System, J. Phase Equilib., 2000, 21, p 287-301
X.P. Su, N.Y. Tang, and J.M. Toguri, Thermodynamic Assessment of the Ni-Zn System, J. Phase Equilib., 2002, 23, p 140-148
J. Miettinen, Thermodynamic Description of the Cu-Ni-Zn System Above 600 C, CALPHAD, 2003, 27, p 263-274
P. Franke and D. Neuschuetz, Ni-Zn (Nickel-Zinc) Binary System, in Part 5: Binary Systems Supplement 1. Landolt-Boernstein - Group IV Physical Chemistry, 2007, p 1-4
J. Chang, S.K. Seo, and H.M. Lee, Phase Equilibria in the Sn-Ni-Zn Ternary System: Isothermal Sections at 200°C, 500°C, and 800°C, J. Electron. Mater., 2010, 39, p 2643-2652
C. Schmetterer, D. Rajamohan, H. Ipser, and H. Flandorfer, The High-Temperature Phase Equilibria of the Ni-Sn-Zn System: Isothermal Sections, Intermetallics, 2011, 19, p 1489-1501
V. Gandova, J. Romanowska, and G. Vassilev, Comparison Between Sn-Ni-Zn Liquid Phase Thermodynamic Assessments Performed by the CALPHAD Method and by Geometrical Models, RMZ M. & G., 2010, 57, p 441-452
Y. Yuan, S. Delsante, D.J. Li, and G. Borzone, The Isothermal Section of the Ni-Sn-Zn Phase Diagram at 873 K, Intermetallics, 2011, 19, p 1646-1650
H. Flandorfer, F. Gehringer, and E. Hayer, Individual Solutions for Control and Data Acquisition with the PC, Thermochim. Acta, 2002, 382, p 77-87
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15, p 317-425
M. Rechchach, A. Sabbar, H. Flandorfer, and H. Ipser, Enthalpies of Mixing of Liquid In-Sn and In-Sn-Zn Alloys, Thermochim. Acta, 2010, 502, p 66-72
V. Gandova, D. Soares, K. Lilova, J.-C. Tedenac, and G.P. Vassilev, Phase Equilibria in the Sn-Zn-Ni System, Int. J. Mater. Res., 2011, 102, p 257-268
V.D. Gandova, P. Broz, J. Buršík, and G.P. Vassilev, Thermochemical and Phase Diagram Studies of the Sn-Zn-Ni System, Thermochim. Acta, 2011, 524, p 47-55
Acknowledgments
This work was performed in the framework of the European Concerted Research Action COST MP0602 project “Advanced Solder Materials for High Temperature Application”. Financial support by the Austrian Science Fund (FWF) under Project No. P21507 is gratefully acknowledged. Yu.P. was supported through a Short Term Scientific Mission within COST MP0602. A.Ya. was supported by a Lise Meitner Fellowship of the Austrian Science Fund (FWF, Project No. M1324).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Plevachuk, Y., Yakymovych, A., Fürtauer, S. et al. The Enthalpies of Mixing of Liquid Ni-Sn-Zn Alloys. J. Phase Equilib. Diffus. 35, 359–368 (2014). https://doi.org/10.1007/s11669-014-0288-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-014-0288-8