Abstract
This work shows that the titanium dioxide coatings obtained by low-pressure cold gas spraying with the use of the sol–gel amorphous TiO2 powder are characterized by photocatalytic activity despite their partial amorphous content. Moreover, the research outcome suggests that the decomposition rate of organic pollutants is enhanced after long-term exposure to moisture. The condensation humidity test is not detrimental to the continuity and integrity of the coating, but the phase composition of coatings changes—with the exposure to water vapor, the portion of the amorphous phase crystallizes into brookite. The mechanism responsible for the conversion of amorphous TiO2 into brookite is attributed to the water-driven dissolution and reprecipitation of TiO6 octahedra. It has been shown that an additional parameter necessary for the stabilization of the brookite is the oxygen depletion of the amorphous structure of titanium dioxide. Considering the results presented in this paper and the advantages of a portable, low-pressure cold spray system for industrial applications, it is expected that TiO2 coatings produced from a sol–gel feedstock powder can be further developed and tested as efficient photocatalysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composition of coatings and the manner of manufacturing determine their ultimate performance. Developing ceramic coating on a metallic substrate is one simple method to improve or give the surface properties, such as self-cleaning, anti-corrosive, or photocatalytic properties while ensuring hardness or wear resistance. Due to increasing environmental pollution, surface properties of metals are insufficient and therefore the photocatalytic activity given by the coating becomes one of the focal points for surface engineering. That is why the manufacturing of titanium dioxide coatings onto various metal substrates such as aluminum (Ref 1), stainless steel (Ref 2,3,4), copper (Ref 5, 6), or titanium alloy (Ref 7) substrates is still an ongoing research topic. When selecting the perfect coating deposition method, a number of factors must be taken into account, among which the most important are: the chemical composition of the substrate and the coating, the desired coating thickness, the shape, size of the substrate, and the scale of the project. In the case of TiO2, this choice is especially demanding due to the correlation of deposition parameters with features determining photocatalytic activity, including the shape and size of grains, the specific surface, and pore structure of the coating, and additionally the type of polymorphic form of titania. Assuming industrial scale of coating formation, thermal spraying methods seem to be a tempting option and indeed, this group is widely employed in the production of TiO2 coatings reducing wear and corrosion of metal substrates or improving their biocompatibility. Still, the production of photocatalytically active coatings poses a challenge because most of the methods in this group rely on the melting (or partial melting) of the feedstock material during deposition. Low-temperature variations of thermal spraying, such as cold spray, are believed to tackle the problem, but despite many attempts: (Ref 8,9,10,11), obtaining ceramic coatings by low-pressure cold spraying (LPCS) is still underdeveloped. Only few papers describe the deposition of TiO2 coatings, and what is more, there is still no consensus on the mechanisms enabling the deposition of ceramic particles. The most frequently proposed mechanism assumes deposition by plastic deformation of a metallic substrate. It has recently been shown that if this mechanism is additionally extended to the deformation of the deposited particles using feedstock in the form of amorphous agglomerates, it is possible to obtain thick, continuous coatings by low-pressure cold spraying (Ref 12, 13). Such an alternative amorphous feedstock powder can be produced by the sol–gel method, which gives an additional chance to control not only the size of the particles and their degree of agglomeration but also allows for additional chemical modification of the produced ceramics (Ref 13). However, despite the undoubted success of the production of the TiO2 coating by the LPCS method, the phase composition allows questioning its photocatalytic use. In (Ref 12), it is shown that spraying of amorphous powder with the air at a pressure of 0.5 MPa and temperature of 600 °C allows only partial crystallization to anatase to form a mixed anatase-amorphous coating. Some works show that a low degree of crystallinity is a factor to reduce photocatalytic activity (Ref 14, 15). Therefore, one could think that annealing after deposition would provide direct crystallization of the amorphous phase. However, high-temperature post-treatment may increase grain coarsening, reduce the specific surface area, or induce possible crystallization of rutile, which in turn may lead to a decrease in photocatalytic activity. Furthermore, any additional treatment becomes an additional production step, prolonging the overall manufacturing process and raising its costs. The single-step LPCS deposition of sol–gel TiO2, presented in this paper, is advantageous considering its simplicity and the ability to provide functionalized ceramic coatings at once. Therefore, the main goal of this work is to investigate whether the obtained low-pressure cold spraying amorphous–anatase TiO2 coatings exhibit photocatalytic activity in the state after deposition—without any additional post-treatments. Another prime motivation for conducting photocatalytic tests of coatings with such a phase composition was the latest literature reports showing the high potential of amorphous titanium dioxide (Ref 16,17,18,19) and mixtures of crystalline polymorphs with amorphous TiO2 (Ref 20, 21) in the processes of photocatalytic decomposition of organic pollutants. The last objective of this research relates to operational characteristics, or more accurately—changes in photocatalytic activity of the tested coatings influenced by long-term exposure to atmospheric humidity. It is commonly known that due to the high hydrophilicity of titanium oxide, water gets easily absorbed in the pore network. Because the outdoor humidity is often very high, condensation can occur between particles and pores of the TiO2 coating. The humid atmosphere influences not only the durability and stability of the coatings under such extreme conditions, but also may induce changes in the phase composition of the coating. Several studies have shown that even at low operating temperatures (room temperature or near room temperature), there may be induced water-assisted crystallization of amorphous TiO2 (Ref 22,23,24,25). In those articles, the condensed water was assumed to act as a catalyst for the rearrangement of randomly distributed TiO6 octahedra and anatase formation (Ref 22,23,24,25) but anywhere other polymorphs (brookite or rutile) appear as a result of crystallization.
The paper presents the effect of long-term exposure of the TiO2 low-pressure cold-sprayed coatings to water vapor. The results of the condensation humidity test evidence the change in the phase composition of the initially anatase–amorphous coating to the anatase–brookite mixture which is indirectly related to the interaction of the coating with water. The photocatalytic activity of samples (before and after the humidity test) demonstrates that despite the presence of the amorphous TiO2 phase in the coating, low-pressure cold-sprayed coatings have the potential as photocatalysts. Additionally, when exposed to long-term water vapor, these coatings significantly increase the photodegradation rate of methylene blue.
Materials and Methods
The self-made TiO2 amorphous sol–gel powder was used as a feedstock powder. The description of its synthesis, by the sol–gel method, was presented in a previous study (Ref 12). Plates of aluminum alloy AA1350 (99.5 wt.% of Al), with dimensions of 20x20x7 mm, were employed as substrates for coating deposition. The substrates surfaces were activated by sandblasting under a pressure of 0.6 MPa using alumina sand—Al2O3 particles of irregular crashed shape and size of 350 μm. The commercial low-pressure cold spray facility DYMET 413 (Obninsk Center for Powder Spraying, Obninsk, Russia), equipped with a single resistance heater located in spraying gun, was used for spraying. A standard circular de Laval nozzle was used (a throat and outlet diameters of 2.5 and 5 mm, respectively). An aerosol powder feeder RBG 1000 D (Palas, Karlsruhe, Germany) powered by nitrogen pressurized to 0.1 MPa was connected at the beginning of the divergent part of the nozzle and fed powder radially, and the powder feeding rate was calculated as approximately 12.8 mg/s. Air with a pressure of 0.5 MPa and a temperature of 600 °C was used as a working gas. The spray distance was set to 10 mm, while the distance between the next spraying beads was 2 mm. A spraying gun was attached to the manipulator (BZT Maschinenbau GmbH, Leopoldshöhe, Germany), moving with a traverse speed of 5 mm/s. Coatings were obtained with one pass of the spray gun.
The morphology of the samples was analyzed using SEM microscope (Hitachi S-3400 N, Tokyo, Japan) equipped with SE, BSE detectors, and EDX system for elemental analysis. The metallographic specimens were prepared by cutting the sample in the middle of its length. Afterward, the cross sections were polished without etching.
The X-ray diffraction measurements were performed on an X-ray diffractometer Ultima IV (Rigaku, Japan), with CuKα irradiation (λ = 1.54056 Å) in the range of angles 2θ from 5° to 70°, a step of 0.05, and exposure time 3 s per point. Raman spectroscopy analysis was carried out using the Raman spectrophotometer LabRam HR800 (Horiba Jobin-Yvon, Japan). Each spectrum was recorded at room temperature with Ar+ laser (514.55 nm) as an excitation source. On the surface of each sample, at least five measurements were made.
The produced TiO2 coatings were tested for resistance to a humid atmosphere in compliance with DIN 50017:1982. Condensation humidity tests, simulating the harmful effects of outdoor humidity, were performed using a controlled atmosphere maintained at 100% relative humidity, at a temperature of 40 °C, to allow condensation on the test samples. The exposure time of the samples to moisture was: 96, 192, 384, 768, and 1000 hours. After the set test time, each of the specimens was subjected to XRD and Raman tests to determine any changes in the phase composition. The selected samples were additionally subjected to microscopic morphological examinations. The resulting samples are referred to throughout the text as TiO2(x) coatings where x in superscript denotes the time of sample exposure to moisture (to be specific: 0, 96, 192, 384, 768, and 1000 h). In this convention, TiO2(0) denotes a sample that has not been exposed to moisture.
The photocatalytic properties of the coatings were evaluated in an aqueous medium by the decomposition of model pollutant—methylene blue (MB). The extent of MB degradation in the solution was quantitatively estimated with UV/VIS Nicolet Evolution 100 spectrophotometer by analyzing the change in the MB absorbance peak at 663 nm. The absorbance values were converted into the dye concentration using the beforehand prepared calibration curve for the MB aqueous solution (absorbance vs. concentration). Absorbance measurements were taken every 30 minutes, up to 5 hours of the experiment, separately for both reactors: the reference reactor without the photocatalyst and photocatalytic reactor containing the sample. To provide a uniform condition for each measurement, the preparation stage included surface pre-treatment, preparation of dye solution, and sample equilibration in the MB solution. Firstly, surfaces of all tested samples were subjected to at least 24-h UVA exposure. In the meantime, the methylene blue solution \(\left({\mathrm{C}}_{\mathrm{M}}=1{10}^{-5} \mathrm{mol}/{\mathrm{dm}}^{3}\right)\) in the demineralized water was prepared, and then, reference and test reactors were filled with 30 ml of the MB solution. Subsequently, tested samples were immersed in the dye solution in the dark for 1 hour to reach adsorption–desorption equilibrium. After reaching equilibrium, each reactor was exposed to UVA radiation provided by a standard strip lamp, equipped with a UVA range bulb model: TL-K40W10R ACTINC. A time of 30 minutes is required for the radiation source to gain the full intensity of UVA irradiation. The distance between the photocatalyst surface and the source of irradiation was 750 mm. At such an arrangement, the UVA light intensity was measured with a standard photodiode power sensor (S120C, Thorlabs) and amounted to 110 µW.
Results and Discussion
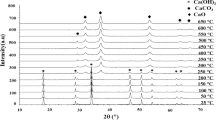
The coatings, sprayed with gas preheated to 600 °C characterized in detail through the structure, morphology, and thermal stability in the previous paper (Figs. 8, 9, 10 in Ref 12), were subjected to the long-term exposure to water vapor. The amorphous–anatase composition of TiO2(0) samples changes in the humid atmosphere (Fig. 1, Fig. 2a). The first difference to the initial state is detected in TiO2(384) as the occurrence of an additional diffraction peak typical for brookite at 2θ about 30.8°. For further measurements, also other brookite peaks become more apparent. From that point, the characteristic hamp ranging roughly from 2 theta 20° to 35°, related to the amorphous content, gradually flatten till the end of the experiment (Fig. 1). The more precise XRD measurement (Fig. 2a) reveals that the hamp practically disappears after 1000 hours of exposure to moisture. This decline indicates the improvement of coatings crystallinity.
Influence of long-term moisture exposure on the structure of the low-pressure cold-sprayed TiO2 coating. (a) XRD patterns and (b) Raman spectra of the TiO2(0) (3 plots at the bottom) and TiO2(1000) (3 plots at the top) samples. The anatase diffraction peaks and bands marked in red, the brookite ones in green.
Raman spectroscopy was employed for better insight into the phase composition of the samples before and after 1000 h of moisture condensation humidity test (Fig. 2b). Besides anatase bands confirmed in the former spectrum (∼147, ∼398, ∼521, and ∼641 cm−1), there appear five additional ones after 1000 h exposure to the moisture. The most intensive Raman band of brookite located at ∼152 cm−1 influences the width of the anatase Raman mode at ∼147 cm−1. There also appear less intense but separate brookite bands at, respectively, ∼247, ∼320, ∼367, ∼401, and ∼457 cm−1 (Fig. 2b). It is important to note that during the process of TiO2(0) spraying, the amorphous feedstock powder undergoes the partial transformation to the form of anatase (Ref 12), but still some share of the amorphous phase is present in the structure of the coating (Fig. 1, 2a). In the literature, the crystallization of TiO2 from the amorphous phase is described as a result of high-temperature annealing (Ref 26). As regards low-temperature alternatives, some other sources reported that it is possible to induce crystallization at room temperature with water or steam (Ref 22,23,24,25). According to the knowledge of authors, crystallization of brookite as a result of moisture or condensed water has not been shown so far—all found reports describe crystallization of anatase. However, the available literature suggests that brookite crystallization could be facilitated by other factors (Ref 27,28,29,30). Most often, it is emphasized that for the crystallization of the brookite, it is necessary to create oxygen vacancies in the vicinity of titanium ions (Ref 27, 28). Such conditions are ensured when the partial pressure of oxygen during synthesis is lowered (Ref 27) or when titanium precursors with Ti3+ valence state (e.g., TiCl3) (Ref 29) are used for the synthesis. A similar effect is also exerted by the introduction of admixtures of ions with a lower oxidation state than Ti4+, called acceptor ions. This is best exemplified by naturally occurring mineral brookite often found with trace amounts of acceptor ions, such as Fe3+ (Ref 27, 31). In the engineering practice, such ions are responsible for the oxygen vacancy formation and thus crystallization of metastable brookite can be introduced on the TiO2 synthesis route (Ref 32). In the case of coatings, those ions can diffuse into the oxide structure from the substrate, for instance, as P. Navotna et al. (Ref 33) have shown due to thermal treatment. To check whether the water vapor influenced the structure of TiO2(1000) coating, SEM microscopic examinations were carried out. Coatings surface topography remains unchanged after the condensation humidity test. Both samples present a typical LPCS coating structure made of partially amorphous TiO2 particles (Fig. S1).
The integrity of the ceramic-type coating and the metallic substrate is a necessity for the sample to retain its functionality, and therefore, detailed observation of the microstructure of both samples was conducted. The SEM micrograph of the sample after 1000 h in the humid atmosphere is shown next to the TiO2(0) structure for better comparison (Fig. 3). TiO2(0) coating (Fig. 3a) is a 250-μm-thick, relatively dense deposit with characteristic packing (the crystalline anatase is randomly mixed with the amorphous phase) (Fig. 3a, area A). As a result of the LPCS deposition process, the crystallites can be found more frequently near the substrate, which was described in the previous paper (Ref 12). When ground, those crystallites are either progressively removed and form a flat surface of cross section or ripped off which results in brittle detachment, observed as darker highly porous regions about the bottom of the initial coating (Fig. 3a, area B).
Sample TiO2(1000) retains successfully the integrity and continuity of the initial state (Fig. 3b) as well as its packing (Fig. 3b, area A). The only significant difference is the formation of the interlayer of thickness up to 5 μm (Fig. 3b, area B). In the thorough inspection of the coating–substrate interface, it is visible that the TiO2(1000) sample is ground more uniformly than the initial sample which evidences better crystallites fitting into the coating and may originate from the interlayer overgrowth, densifying the bottom of the coating.
To observe the element composition of the coating–substrate interface, the EDX point analysis was carried out (Fig. 4). It was found out that the interlayer consists of Al and O atoms. Aluminum atoms are detected also within the TiO2 coating. Further from the interface, the content of aluminum is lower. Taking into consideration that coating is deposited on an aluminum alloy, it can be concluded that the interlayer is a passivation layer of the substrate material. The 1000-h stay in the elevated temperature of the substrate throughout condensation humidity tests (about 40 °C) and the accumulated condensed water infiltrating TiO2 coating deep down to the substrate interface could quicken the aluminum passivation process. The formation of the interlayer is profitable due to several aspects: It protects the base material and provides the substrate integration with deposited coating due to the overgrowth through the TiO2 coating. The EDX-detected presence of aluminum in the coating suggests either the built-up of the passivation layer or diffusion of Al through the coatings. The first option is more evident, but to establish whether aluminum diffused other tests are needed.
The diffusion of ions from the metallic substrate into the TiO2 coating has been observed and described in various papers (Ref 34–36). The authors of these studies suggested that in the case of the stainless steel substrate, Fe3+ ions from the passive layer diffuse into the crystalline TiO2 coating and form separate Fe3O4 nanoparticles in it. This phase is responsible for the reduction of photocatalytic activity by acting as recombination centers in the TiO2 structure. Different observations were made in the case of the diffusion of sodium ions from glass substrates, and the separate oxide phases in the TiO2 coating were not detected, which was attributed to the low crystallinity of this coating (Ref 30,37). In the case described in this paper, the low-pressure cold-sprayed TiO2 coating consists of two phases, anatase, and amorphous phase. Al3+ ions from the substrate (or actually from the passivation layer at the interface between the substrate and the coating) can diffuse much more easily in the amorphous phase, substituting in an octahedral sites Ti4+ ions withÛius \(\left({r}_{{Al}^{3+}}=0.535 \text{\AA},{ r}_{{Ti}^{4+}}=0.605 \text{\AA}\right)\) (Ref 38). Thus, the introduction of Al3+ ions with a lower valence than Ti4+ ions creates an oxygen deficiency in amorphous titanium dioxide which enables the system to balance the charge. It seems clear that the generation of oxygen defects in amorphous TiO2 is a predisposing factor for the crystallization of brookite, but may be not sufficient. Energy is needed to organize the oxygen-depleted structure of the oxide, but the temperature used in this work during the condensation test is too low to induce crystallization. Thus, it must be recognized that the assistance of water molecules from moisture is the necessary factor responsible for crystallization. However, the mechanisms describing the water-assisted crystallization of amorphous TiO2 at room temperature in the literature show that the anatase order is preferred (Ref 23, 25). This leads to the conclusion that, evidently, in this work, the crystallization of brookite must be related to the interaction of two factors at the same time, namely oxygen defects of amorphous TiO2 and the effect of moisture on this specific system.
Both amorphous TiO2 and crystalline forms of TiO2 are composed of TiO6 octahedra, and the process of crystallization is based on the rearrangement of these units. In a crystal structure, the octahedra are ordered by edges or corners sharing (Fig. 5a). It is important to know that corners sharing is preferred because this arrangement of octahedra in the space minimizes the repulsive Coulomb forces between ions with the same charge. As a result, more octahedrons connect each other through the corners in the crystal structure of the stable rutile (Fig. 5b) than in anatase (Fig. 5d), while more octahedra connect by sharing the common oxygen edges in anatase than in rutile. Brookite is midway between anatase and rutile in terms of TiO6 units edges–corners bonding (Fig. 5c). In the sol–gel amorphous TiO2 (Fig. 5e), the TiO6 units are not ordered in space and, importantly, some of them are partially hydrolyzed. (Titanium ions in the octahedron are sometimes bound to hydroxyl groups.) The nucleation and growth processes of anatase, brookite, or rutile occur through the dehydration and cross-linking between adjacent octahedra with the assistance of water (Fig. 5f). In the first stage of the process, a water molecule, using two lone electron pairs, creates a bridge between two –OH groups of adjacent octahedra. This interaction creates a new water molecule by the detachment of the hydroxyl (–OH) group from one octahedron and the hydroxyl proton (H+) from the second octahedron. The oxygen atom remaining after detaching the hydroxyl proton combines with the second octahedron where the –OH group is eliminated what leads to the formation of edge-sharing octahedra. The more –OH groups are adjacent to each other, the greater the tendency to edge-sharing of symmetrical octahedrons and thus to the formation of a crystalline form of anatase. For this reason, the anatase structure consists mainly of edge-sharing octahedrons. Note that in the case described in this work, some octahedra are oxygen deficient which is directly responsible for the increased probability of TiO6 octahedral rearrangements from the edge-sharing to the corner-sharing octahedra (Fig. 5g). It can be assumed that in the case of significant oxygen deficiency, this increased tendency to create corner-shared octahedra leads to rutile crystallization; however, when the deficiency is not so high, the resulting crystal order will be typical for brookite.
Schematic representation of structural reorganization of TiO6 octahedral connectivity and configuration (a) with an indication of octahedra connected by edges or corners. Crystal structure of (b) rutile, (c) brookite, (d) anatase, and (e) amorphous phase. (f) Formation of edge-sharing connection from corner-shared TiO6 octahedra containing residual hydroxyl groups in the water environment. (g) Formation of edge-sharing connection from corner-shared TiO6 octahedra due to migration of oxygen vacancies.
At this point, being aware of all the changes in the phase composition of the investigated coatings, the question arises about their photocatalytic activity. It is commonly known that not only the phase composition determines the photocatalytic potential (Ref 15,16,18–21). For this reason, it was decided to carry out tests to check the catalytic activity of TiO2(0) coatings in the reactions of decomposition of the model pollutant under the influence of UV radiation for 4 h. The absorption spectra of MB measured throughout the test evidence the changes in dye concentration (blue plot in Fig. 6a). For the reference reactor, no change was observed. In the TiO2 sample-containing reactor, there is a visible decrease in the peak intensity of the azo bond (663 nm) assisted by fading of MB solution. The analysis of the entire range of the time-dependent UV–Vis absorption spectra shows that the intensity of all four MB absorption bands decreases gradually, without revealing any new band in the spectra confirming lack of reaction by-product, and therefore, as the degradation is stable, only MB photodegradation rate curve is presented (Fig. 6). Based on the change of the intensity of the peak centered at 663 nm, the UV degradation rate of the dye was plotted as \(\frac{{C}_{x}}{{C}_{0}}\) versus time (where \({C}_{0}\) and \({C}_{x}\) are the initial concentration and the concentration of dye after different time of photo irradiation, respectively.) As can be seen on the red plot, TiO2(0) coating strongly adsorbs MB under dark (red plot, Fig. 6a). The removal of the dye due to the coating adsorption after one hour was about 14%. The UV degradation efficiency of methylene blue using TiO2(0) cold-sprayed catalyst was calculated to be about 22% after 4 hours of UV exposure. The MB removal rate by TiO2(1000) under simulated UV illumination proceeded similarly (green plot, Fig. 6a)—after one hour was about 16% which was only slightly different from the 14% of adsorption observed with the TiO2(0) coating. However, it was calculated that the degradation efficiency of methylene blue after 4 hours of UV irradiation with TiO2(1000) catalyst was about 34%. The total dye loss due to adsorption and 4-hour photocatalytic degradation in the TiO2(1000) case was estimated to be around 50%, which is also much better than the total 36% achieved by TiO2(0). To investigate whether there are any changes in microstructure as a result of the 5-hour immersion in the liquid MB (1h of the dark adsorption and 4h—photocatalytic process), both TiO2(0) and TiO2(1000) samples were examined with SEM microscope. No changes in the structure morphology were observed (Fig. S2).
(a) MB photodegradation rate under UVA irradiation in the presence of TiO2(0) and TiO2(1000). The UVA light was turned on after 60 min dark adsorption—dark; (b) pseudo-first-order kinetics and apparent rate constant for the MB photodegradation processes using TiO2(0) (red) and TiO2(1000) (green) photocatalysts
Not only the degradation efficiency but also MB decomposition kinetics was determined (Fig. 6b). The shape of both curves is in good agreement with the results of other works (Ref 39–41) and suggested pseudo-first-order kinetic rate plot which can be described by the widely accepted in heterogeneous photocatalysis Langmuir–Hinshelwood kinetic model: \(\mathrm{ln}\left(\frac{{C}_{0}}{{C}_{x}}\right)=kt\), where \({C}_{0}\) is the concentration of the dye after darkness adsorption, \({C}_{x}\) is the concentration of the dye measured at the time point \(t\), \(k\) refers to the apparent rate constant (min-1), and \(t\) (min) is the irradiation time. The degradation rate constants (\(k\) value) were calculated to be 0.00129 min-1 (red plot, Fig. 6b) and 0.00217 min-1 (green plot, Fig. 6b), respectively, for TiO2(0) and TiO2(1000) samples. The 59% enhancement in pseudo-first-order kinetics for the degradation MB proves that changes in the phase composition have a positive effect on the photocatalytic activity of the photocatalyst.
It seems that the most important factor responsible for enhancing the photocatalytic activity is the increase in the crystallinity of the coating structure. After 1000 h of the test, due to the crystallization-catalyzing action of water molecules, the share of the amorphous phase significantly decreases and the brookite crystallizes. Crystallization of the amorphous part of the coating to the brookite form was related to the choice of the substrate. The partial oxidation of the aluminum and possible diffusion of ions into the coating resulted in a partial deoxidation of the TiO6 octahedra that favored the brookite phase. Selecting a different substrate material may encourage the amorphous phase in LPCS coatings under high humidity conditions to crystallize in the anatase phase. Also, it is difficult to indicate at the present stage of the research what phase composition of the coating would be more favorable for photocatalytic activity. In the literature, both the increase in crystallinity and the appearance of a mixture of TiO2 polymorphs are factors favorably influencing the ability to catalyze the processes of decomposition of organic pollutants under irradiation (Ref 32, 42,43,44,45). Apart from the phase composition itself, more factors are important in photocatalytic processes (e.g., active surface). However, the aim of the work, which was to show that the LPCS technique is a convenient approach for forming the photocatalytically active TiO2 coatings from amorphous sol–gel feedstock powders, was achieved. The partial amorphous nature of the coatings and their relatively low degree of compactness do not limit their commercial application. Under operating conditions assuming high humidity, these apparent limitations are directly responsible for the increase in the degree of crystallinity and thus for the improvement of photocatalytic activity.
Conclusions
In this study, the photocatalytic activity of the low-pressure cold-sprayed anatase–amorphous TiO2 coatings was tested toward methylene blue (MB) dye aqueous solution as a pollutant model. In addition, it was also studied how the long-term operation of such coatings in a high humidity environment will affect its photocatalytic activity. For this purpose, the coatings were subjected to a standardized condensation humidity test. The morphological and structural tests carried out after 1000 hours revealed that these extreme test conditions led to further crystallization (brookite forming), maintaining continuity and enhanced integration of coatings with the aluminum substrate. More importantly, a significant improvement in photocatalytic activity was observed as a result of the loss of amorphicity. The conducted research shows that the coatings sprayed with sol–gel powders using the low-pressure cold spray method show potential in photocatalytic applications. The selection of the feedstock material in the form of a sol–gel amorphous powder not only determined the success of the coating deposition but also retained partial amorphicity of the feedstock material which then was susceptible to phase transformation in the humidity chamber. In that sense, the wet conditions no longer pose a threat but enhance the photocatalytic activity in the working environment.
Change history
01 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11666-022-01430-z
References
M Mesbah M Sarraf A Dabbagh B Nasiri-Tabrizi S Paria SM Banihashemian AR Bushroa G Faraji T Tsuzuki HR Madaah Hosseini 2020 Synergistic Enhancement of Photocatalytic Antibacterial Effects in High-Strength Aluminum/TiO2 Nanoarchitectures Ceram. Int. 46 15 24267 24280
G Balasubramanian DD Dionysiou MT Suidan V Subramanian I Baudin JM Laîné 2003 Titania Powder Modified Sol-Gel Process for Photocatalytic Applications J. Mater. Sci. 38 4 823 831
Y Chen DD Dionysiou 2006 TiO2 Photocatalytic Films on Stainless Steel: The Role of Degussa P-25 in Modified Sol-Gel Methods Appl. Catal. B: Environ. 62 3–4 255 264
L Zhang Y Zhu Y He W Li H Sun 2003 Preparation and Performances of Mesoporous TiO2 Film Photocatalyst Supported on Stainless Steel Appl. Catal. B: Environ. 40 4 287 292
CJ Chung CC Chiang CH Chen CH Hsiao HI Lin PY Hsieh JL He 2008 Photocatalytic TiO2 on Copper Alloy for Antimicrobial Purposes Appl. Catal. B: Environ. 85 1–2 103 108
DM Chun MH Kim JC Lee SH Ahn 2008 TiO2 Coating on Metal and Polymer Substrates by Nano-Particle Deposition System (NPDS) CIRP Ann. - Manuf. Technol. 57 1 551 554
K Schmidt S Buhl N Davoudi C Godard R Merz I Raid E Kerscher M Kopnarski C Müller-Renno S Ripperger J Seewig C Ziegler S Antonyuk 2017 Ti Surface Modification by Cold Spraying with TiO2 Microparticles Surf. Coatings Technol. 309 749 758
J Morimoto T Onoda Y Sasaki N Abe 2004 Improvement of Solid Cold Sprayed TiO2-Zn Coating with Direct Diode Laser Vacuum 73 3–4 527 532
XL He GJ Yang CJ Li CX Li SQ Fan 2014 Room Temperature Cold Sprayed TiO2 Scattering Layer for High Performance and Bending Resistant Plastic-Based Dye-Sensitized Solar Cells J. Power Sources 251 122 129
M Robotti S Dosta C Fernández-Rodríguez MJ Hernández-Rodríguez IG Cano EP Melián JM Guilemany 2016 Photocatalytic Abatement of NOx by C-TiO 2 /Polymer Composite Coatings Obtained by Low Pressure Cold Gas Spraying Appl. Surf. Sci. 362 274 280
M Yamada H Isago H Nakano M Fukumoto 2010 Cold Spraying of TiO 2 Photocatalyst Coating with Nitrogen Process Gas J. Therm. Spray Technol. 19 6 1218 1223
A Baszczuk M Jasiorski M Winnicki 2018 Low-Temperature Transformation of Amorphous Sol-Gel TiO2 Powder to Anatase During Cold Spray Deposition J. Therm. Spray Technol. 27 8 1551 1562 https://doi.org/10.1007/s11666-018-0769-0
A Gibas A Baszczuk M Jasiorski M Winnicki 2019 Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings Coatings 9 12 829
J Lee J Hwang H Park T Sekino W-B Kim 2020 Preparation of Ultra-Thin TiO2 Shell by Peroxo Titanium Complex (PTC) Solu- Tion-Based Green Surface Modification, and Photocatalytic Activity of Homo- Core/Shell TiO2 Appl. Surf. Sci. 540 148399
SP Mulakov PM Gotovtsev AA Gainanova GV Kravchenko GM Kuz’micheva VV Podbel’skii 2020 Generation of the Reactive Oxygen Species on the Surface of Nanosized Titanium(IV) Oxides Particles under UV-Irradiation and Their Connection with Photocatalytic Properties J. Photochem. Photobiol. A Chem. 393 1124
S Sun P Song J Cui S Liang 2019 Amorphous TiO 2 nanostructures: synthesis, fundamental properties and photocatalytic applications Catal. Sci. Technol. 9 16 4198 4215
H Sabbah 2017 Effect of Sputtering Parameters on the Self-Cleaning Properties of Amorphous Titanium Dioxide Thin Films J. Coat. Technol. Res. 14 6 1423 1433
C Randorn JTS Irvine P Robertson 2008 Synthesis of Visible-Light-Activated Yellow Amorphous TiO2 Photocatalyst Int. J. Photoenergy https://doi.org/10.1155/2008/426872
J Huang Y Liu L Lu L Li 2012 The Photocatalytic Properties of Amorphous TiO 2 Composite Films Deposited by Magnetron Sputtering Res. Chem. Intermed. 38 487 498
J Lyu J Shao Y Wang Y Qiu J Li T Li Y Peng F Liu 2019 Construction of a Porous Core-Shell Homojunction for the Photocatalytic Degradation of Antibiotics Chem. Eng. J. 358 614 620
B Ohtani Y Ogawa S Nishimoto 1997 Photocatalytic Activity of Amorphous-Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions J. Phys. Chem. B 101 3746 3752
J Su X Zou GD Li YM Jiang Y Cao J Zhao JS Chen 2013 Room-Temperature Spontaneous Crystallization of Porous Amorphous Titania into a High-Surface-Area Anatase Photocatalyst Chem. Commun. 49 74 8217 8219
MK Hossain US Akhtar AR Koirala IC Hwang KB Yoon 2015 Steam-Assisted Synthesis of Uniformly Mesoporous Anatase and Its Remarkably Superior Photocatalytic Activities Catal. Today 243 228 234
X Wang D Zhang Q Xiang Z Zhong Y Liao 2018 Review of Water-Assisted Crystallization for TiO2 Nanotubes Nano-Micro Lett. https://doi.org/10.1007/s40820-018-0230-4
N Li Q Zhang JB Joo Z Lu M Dahl Y Gan Y Yin 2016 Water-Assisted Crystallization of Mesoporous Anatase TiO2 Nanospheres Nanoscale, Royal Soc. Chem. 8 17 9113 9117
H Albetran IM Low 2019 Parameters Controlling the Crystallization Kinetics of Nanostructured TiO2 - An Overview Mater. Today Proc. 16 25 35 https://doi.org/10.1016/j.matpr.2019.05.279
JS Mangum O Agirseven JES Haggerty JD Perkins LT Schelhas DA Kitchaev LM Garten DS Ginley MF Toney J Tate BP Gorman 2018 Selective Brookite Polymorph Formation Related to the Amorphous Precursor State in TiO2 Thin Films J. Non. Cryst. Solids 2019 505 109 114 https://doi.org/10.1016/j.jnoncrysol.2018.10.049
JS Mangum LM Garten V Jacobson DS Ginley BP Gorman 2019 Exploring the Link Between Amorphous Structure and Crystallization Behavior of Titania Thin Films by Electron-Based Pair Distribution Functions and in-Situ TEM Microsc. Microanal. Politechnika Wroclawska CWINT 25 S2 1506 1507
V Štengl S Bakardjieva N Murafa J Šubrt H Měšťánková J Jirkovský 2007 Preparation, Characterization and Photocatalytic Activity of Optically Transparent Titanium Dioxide Particles Mater. Chem. Phys. 105 1 38 46
H Xie N Li B Liu J Yang X Zhao 2016 Role of Sodium Ion on TiO2 Photocatalyst: Influencing Crystallographic Properties or Serving as the Recombination Center of Charge Carriers? J. Phys. Chem. C 120 19 10390 10399
“https://www.mindat.org/min-787.html; Last Visited 1 March 2021,” n.d.
A Paola Di M Bellardita L Palmisano 2013 Brookite, the Least Known TiO2 Photocatalyst Catalysts 3 1 36 73
P Novotna J Krysa J Maixner P Kluson P Novak 2010 Photocatalytic Activity of Sol-Gel TiO2 Thin Films Deposited on Soda Lime Glass and Soda Lime Glass Precoated with a SiO2 Layer Surf. Coatings Technol. 204 16–17 2570 2575 https://doi.org/10.1016/j.surfcoat.2010.01.043
Y Zhu L Zhang L Wang Y Fu L Cao 2001 The Preparation and Chemical Structure of TiO2 Film Photocatalysts Supported on Stainless Steel Substrates via the Sol-Gel Method J. Mater. Chem. 11 7 1864 1868
JH Pan Z Lei WI Lee Z Xiong Q Wang XS Zhao 2012 Mesoporous TiO 2 Photocatalytic Films on Stainless Steel for Water Decontamination Catal. Sci. Technol. 2 1 147 155
AF Gualdrón-Reyes AM Meléndez I González L Lartundo-Rojas ME Niño-Gómez 2018 Effect of Metal Substrate on Photo(Electro)Catalytic Activity of B-Doped Graphene Modified TiO2 Thin Films: Role of Iron Oxide Nanoparticles at Grain Boundaries of TiO2 J. Phys. Chem. C 122 1 297 306
HJ Nam T Amemiya M Murabayashi K Itoh 2004 Photocatalytic Activity of Sol-Gel TiO2 Thin Films on Various Kinds of Glass Substrates: The Effects of Na+ and Primary Particle Size J. Phys. Chem. B 108 24 8254 8259
RD Shannon 1976 Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides Acta Cryst. A32 751 767
D Komaraiah E Radha J Sivakumar MV Ramana Reddy R Sayanna 2020 Photoluminescence and Photocatalytic Activity of Spin Coated Ag+ Doped Anatase TiO2 Thin Films Opt. Mater. (Amst) 108 August 110401 https://doi.org/10.1016/j.optmat.2020.110401
E Assayehegn A Solaiappan Y Chebude E Alemayehu 2019 Fabrication of Tunable Anatase/Rutile Heterojunction N/TiO2 Nanophotocatalyst for Enhanced Visible Light Degradation Activity Appl. Surf. Sci. 2020 515 145966 https://doi.org/10.1016/j.apsusc.2020.145966
U Chinonso O Ibukun H Kyung Jeong 2020 Air Plasma Treated TiO2/MWCNT Composite for Enhanced Photocatalytic Activity Chem. Phys. Lett. 757 August 137850 https://doi.org/10.1016/j.cplett.2020.137850
J Yu B Wang 2010 Effect of Calcination Temperature on Morphology and Photoelectrochemical Properties of Anodized Titanium Dioxide Nanotube Arrays Appl. Catal. B Environ. 94 3–4 295 302
S Hotchandani PV Kamat 1992 Charge-Transfer Processes in Coupled Semiconductor Systems. Photochemistry and Photoelectrochemistry of the Colloidal CdS-ZnO System J. Phys. Chem. 96 16 6834 6839
K Fischer A Gawel D Rosen M Krause AA Latif J Griebel A Prager A Schulze 2017 Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis Catalysts 7 7 209
JM Wu B Huang 2007 Enhanced Ability of Nanostructured Titania Film to Assist Photodegradation of Rhodamine B in Water through Natural Aging J. Am. Ceram. Soc. 90 1 283 286
Acknowledgements
The research was financed by the Department of Mechanics, Materials and Biomedical Engineering, Wroclaw University of Science and Technology, under contract no. 8211104160 K58W10D07. Additional support was provided by the Polish National Science Centre under contract no. 2016/23/D/ST8/00675 (Project title: The mechanism of joining submicron ceramic particles in cold spraying process).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: All the author names were listed incorrectly in the PDF version, with the last names appearing before the first names. For example, “Seremak Wioletta” should be “Wioletta Seremak”. The names were also switched in the XML, resulting in an incorrect citation of the authors’ names in the publication website (SpringerLink). For example, “Wioletta, S.” should be “Seremak, W.”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seremak, W., Baszczuk, A., Jasiorski, M. et al. Photocatalytic Activity Enhancement of Low-pressure Cold-Sprayed TiO2 Coatings Induced by Long-term Water Vapor Exposure. J Therm Spray Tech 30, 1827–1836 (2021). https://doi.org/10.1007/s11666-021-01244-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-021-01244-5