Abstract
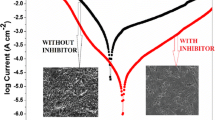
The corrosion inhibition of mild steel in 1 M hydrochloric acid by a few piperidin-4-one oxime derivatives, namely, 1,3-dimethyl-2,6-diphenyl piperidin-4-one oxime (I), 3,3-dimethyl-2,6-diphenyl piperidin-4-one oxime (II), and 3-isopropyl-2,6-diphenyl piperidin-4-one oxime (III) was studied using chemical weight loss method, electrochemical polarization and impedance spectroscopy, SEM with EDS, XRD, FT-IR measurements, and semi-empirical AM1 method for electronic properties. The weight loss measurements at four different temperatures such as 30, 40, 50, and 60 °C showed that the percentage inhibition efficiency (IE) of these compounds increased with increase of concentration and decreased with increase of temperature. The IE followed the order III < II < I. It was found that these inhibitors function through physical adsorption mechanism obeying Temkin’s isotherm. Polarization studies showed that these compounds act as mixed-type inhibitors. Impedance measurements revealed the increase of charge transfer resistance with inhibitor concentration. Surface analysis using SEM, XRD, and FT-IR revealed the formation of protective film over the mild steel surface. The electronic properties calculated using AM1 semi-empirical method explained the inhibition characteristics. The quantum chemical studies showed that ring nitrogen and phenyl rings are the probable active centers to inhibit corrosion process.










Similar content being viewed by others
References
P. Bothi Raja and M.G. Sethuraman, Atropine Sulphate as Corrosion Inhibitor for Mild Steel in Sulphuric Acid Medium, Mater. Lett., 2008, 62(10–11), p 1602–1604
P. Bothi Raja and M.G. Sethuraman, Inhibitive Effect of Black Pepper Extract on the Sulphuric Acid Corrosion of Mild Steel, Mater. Lett., 2008, 62(17–18), p 2977–2979
A.N. Senthilkumar and M.G. Sethuraman, Corrosion Inhibition Potential of Sulfadimidine, Corros. Rev., 2008, 26(1), p 23–37
N. Hackerman and R.M. Hurd, Proceedings of International Congress on Metallic Corrosion, Butterworth, London, 1962, p 166
S. Muralidharan, K.L.N. Phani, S. Pitchumani, S. Ravichandran, and S.V.K. Iyer, Polyamino-Benzoquinone Polymers: A New Class of Corrosion Inhibitors, J. Electrochem. Soc., 1995, 142(5), p 1478–1483
J. Jayabharathi, A. Manimekalai, T. Consalata Vani, and M. Padmavathy, Synthesis, Stereochemistry and Antimicrobial Evaluation of t(3)-Benzyl-r(2), c(6)-Diaryl-piperidin-4-one and Its Derivatives, Eur. J. Med. Chem., 2007, 42(5), p 593–605
V. Baliah and C.R. Noller, The Preparation of Some Piperidine Derivatives by the Mannich Reaction, J. Am. Chem. Soc., 1948, 70(11), p 3853–3855
A.K. Maayta and N.A.F. Rawashdeh, Inhibition of Acidic Corrosion of Pure Aluminium by Some Organic Compounds, Corros. Sci., 2004, 46, p 1129–1140
E.E. Ebenso, P.C. Okafer, and U.F. Ekpe, Studies on the Inhibition of Aluminium Corrosion by 2-Acetylpheno Thiazine in Chloro Acetic Acid, Anti-Corros. Meth. Mater., 2003, 50(6), p 414–421
S. Muralidharan, M.A. Quraishi, and S.V.K. Iyer, The Effect of Molecular Structure on Hydrogen Permeation and the Corrosion Inhibition of Mild Steel in Acidic Solutions, Corros. Sci., 1995, 37(11), p 1739–1750
S. Rengamani, S. Muralidharan, M. Anbukulandainathan, and S.V.K. Iyer, Inhibiting and Accelerating Effects of Amino Phenols on the Corrosion and Permeation of Hydrogen Through Mild Steel in Acidic Solutions, J. Appl. Electrochem., 1994, 24(4), p 355–360
M. Elachouri, M.S. Hajji, M. Salem, S. Kertit, J. Aride, R. Coudert, and E. Essassi, Some Nonionic Surfactants as Inhibitors on the Corrosion of Iron in Acid Chloride Solutions, Corrosion, 1996, 52(2), p 103–108
B.V. Savithri and S. Mayanna, Tetra Butyl Ammonium Iodide Cetyl Pyridinium Bromide and Cetyl Tri Methyl Ammonium Bromide as Corrosion Inhibitors for Mild Steel in Sulphuric Acid Medium, Ind. J. Chem. Technol., 1996, 3(5), p 256–258
S.Z. Duan and Y.L.Tao, Interface Chemistry, Higher Education Press, Beijing, 1990, p 124–126
F.M. Bayoumi and W.A. Ghanem, Corrosion Inhibition of Mild Steel Using Naphthalene Disulfonic Acid, Mater. Lett., 2005, 59(29–30), p 3806–3809
A.N. Senthilkumar, K. Tharini, and M.G. Sethuraman, Corrosion Inhibitory Effect of Few Piperidin-4-one Oximes on Mild Steel in Hydrochloric Acid Medium, Surf. Rev. Lett., 2009, 16(1), p 141–147
P. Bothi Raja and M.G. Sethuraman, Inhibition of Corrosion of Mild Steel in Sulphuric Acid Medium by Calotropis procera, Pig. Res. Technol., 2009, 38(1), p 33–37
P. Bothi Raja and M.G. Sethuraman, Corrosion Inhibition of Mild Steel by Datura metel (Leaves) in Acidic Medium, Pig. Res. Technol., 2005, 34(6), p 327–331
P. Bothi Raja and M.G. Sethuraman, Studies on the Inhibitive Effect of Datura stramonium Extract on the Acid Corrosion of Mild Steel, Surf. Rev. Lett., 2007, 14(6), p 1157–1164
H. Ashassi-Sorkhabi, B. Shaabani, and D. Seifzadeh, Effect of Some Pyrimidinic Shciff Bases on the Corrosion of Mild Steel in Hydrochloric Acid Solution, Electrochim. Acta, 2005, 50(16–17), p 3446–3452
E. McCafferty and N. Hackerman, Double Layer Capacitance of Iron and Corrosion Inhibition with Polymethylene Diamines, J. Electrochem. Soc., 1972, 119, p 146–154
M. Lagrenee, B. Mernari, M. Bouanis, M. Traisnel, and F. Bentiss, Study of the Mechanism and Inhibiting efficiency of 3,5-Bis(4-methyl thiophenyl)-4H-1,2,4-triazole on Mild Steel Corrosion in Acid Media, Corros. Sci., 2002, 44(3), p 573–588
M. Ozcan and I. Dehri, Electrochemical and Quantumchemical Studies on Some Sulphur Containing Organic Compounds as Inhibitors for the Acid Corrosion of Mild Steel, Prog. Org. Coat., 2004, 51(3), p 181–187
A. Lgamri, H. Abou El Makarim, A. Guenbour, A. Ben Bachir, L. Aries, and S. El Hajjaji, Electrochemical Study of the Corrosion Behaviour of Iron in Presence of New Inhibitor in 1 M HCl, Prog. Org. Coat., 2003, 48, p 63–70
A.A. Rahim, E. Rocca, J. Steinmetz, M.J. Kassim, R. Adnan, and M. Sani Ibrahim, Mangrove Tannins and Their Flavanoid Monomers as Alternative Steel Corrosion Inhibitors in Acidic Medium, Corros. Sci., 2007, 49(2), p 402–417
N. Khalil, Quantum Chemical Approach of Corrosion Inhibition, Electrochim. Acta, 2003, 48(18), p 2635–2640
J. Vosta and N. Hackerman, The Dependence of Capacitance on Time in an Acid Inhibition Corrosion Process, Corros. Sci., 1990, 30(8–9), p 949–950
J. Fang and L. Jie, Quantum chemistry Study on the Relationship Between Molecular Structure and Corrosion Inhibition of Amides, J. Mol. Struct., 2002, 593(1–3), p 179–185
G. Klopman, Chemical Reactivity and the Concept of Charge and Frontier Controlled Reactions, J. Am. Chem. Soc., 1968, 90(2), p 223–234
W.B. Jensen, The Lewis Acid-Base Definitions: A Status, Chem. Rev., 1978, 78(1), p 1–22
K. Aramaki, T. Mochizuki, and H. Nishihara, Impedance Study on Inhibition and Stimulation of Iron Corrosion in Acid Solution by Various Inorganic Anions and Tetra-alkyl ammonium Cation, J. Electrochem. Soc., 1988, 135(6), p 1364–1369
R.G. Pearson, Recent Advances in the Concept of Hard and Soft Acids and Bases, J. Chem. Educ., 1987, 64(7), p 561–567
P. Mutombo and N. Hackerman, The Effect of Some Organo Phosphorous Compounds on the Corrosion Behaviour of Iron in 6 M HCl, Anti-Corros. Meth. Mater., 1998, 45(6), p 413–418
Acknowledgments
The authors thank the authorities of Gandhigram Rural University for their help and encouragement. The authors acknowledge the help received from Dr. Vijayalakshmi, Dr. Parameswaran, and Ms. Radhika, PMD Division, IGCAR, Kalpakkam and for the permission to use the instrumental facilities. Authors (ANS and KT) thank the authorities of SASTRA University, Thanjavur for the support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthilkumar, A.N., Tharini, K. & Sethuraman, M.G. Studies on a Few Substituted Piperidin-4-one Oximes as Corrosion Inhibitor for Mild Steel in HCl. J. of Materi Eng and Perform 20, 969–977 (2011). https://doi.org/10.1007/s11665-010-9719-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-010-9719-9