Abstract
The structural and electrical properties of the xP2O5-(40 − x) Bi2O3-60V2O5 (0 ≤ x ≤ 20) glass system have been investigated. The samples were prepared by the conventional melt-quenching technique. X-ray diffraction (XRD) patterns confirmed the amorphous nature of the present glasses. Nanocrystalline grains were found due to the annealing of the glass samples under study. Nanocrystals with an average grain size of 22 nm were implanted in the glass structure and estimated from the XRD patterns of the glass–ceramic samples. DC conductivity of the glass system has been determined in the temperature range 300–500 K. It was found that the general behavior of electrical conductivity was similar for all the glass compositions and found to decrease with increasing phosphate content. The electrical conductivity of the glass–ceramic nanocrystals obtained by annealing at crystallization temperature (Tc) was much higher than the initial glass. The activation energy (W) was enhanced by annealing and was obtained from plots of temperature-dependent DC conductivity, and found to be 0.23–0.31 eV for glasses and 0.19–0.23 eV for the glass–ceramic nanocrystals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last three decades, because of prospective applications of different glasses, several attempts were made to develop their conduction. Glassy materials have many technological applications (fuel cells, new solid-state batteries, “smart windows”, sensors) due to the large available composition ranges and inherent advantages.1 Vanadium pentoxide (V2O5) has interesting applications, such as optical switching devices, memory switching, and the electrical threshold, because of their thermal stability, relatively high electrical conductivity, and glass formation range compared to other transition metal oxide glasses.2 Transition metal oxides such as P2O5, V2O5, etc. can form glasses when mixed with glass-forming oxides. The vanadate glasses dc conduction and transport properties can be explained by the small polarons hopping model.3 The strong interaction of ions with electrons produces the formation of polarons and the localization in the vanadium glasses.4,5,6,7,8 On the other hand, Bi2O3 is a heavy metal oxide glass, that has attracted considerable attention owing to the wide range of applications in different fields, including thermal and mechanical sensors, glass ceramics, layers for optical, reflecting windows, and optoelectronic devices, etc.9 Vanidate glasses have low melting, high thermal conductivity and, moreover, high thermal expansion coefficients over other glasses. Different heavy metal oxides can be added to vanidate glasses to improve the physical properties.10 In view of the fact that borophosphate glasses are known to be chemically more durable than pure phosphate and borate glasses they have attractive applications.11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 In the present study, considering that V2O5 is a glass network former, by using x-ray diffraction (XRD) and electrical properties of 60V2O5-xP2O5-(40-x)Bi2O3 glasses and glass–ceramic nanocrystals were studied in the temperature range 300–500 K, these types of glasses and glass–ceramic nanocrystals have different applications.
Experimental Method
Samples under investigation were prepared by a melting quenching technique according to the 60V2O5-xP2O5-(40-x)Bi2O3 (mol%) system, where (0 ≤ x ≤ 20). The raw materials were mixed and melted for about 2 h in porcelain crucibles that were placed in an electrically heated furnace at 1475 k, until liquid was formed without any bubbles. The product melt was quickly cooled at room temperature by pouring between two copper plates and pressing it. The prepared glass samples were cut into the desired size and polished. Some of the prepared glass samples were put in an electrically heated furnace at the crystallization temperature (Tc). To identify the structure of the glasses and glass–ceramics, x-ray diffraction (XRD) patterns were measured using a Philips P.W.1390 diffractometer with a Cu Kα target and Ni filter. The recorded diffraction data were obtained for 2θ between 10° and 80°. The dc conductivity (σ) of the prepared glasses and glass–ceramics were measured at temperatures between 300 and 500 K using two probe methods. The polished samples were deposited with silver paste electrodes on both surfaces. The dc data were collected by using a multimeter (Keithley 760), hence, the I–V characteristic between the electrodes was determined.
Results and Discussion
X-ray Diffraction (XRD) measurement
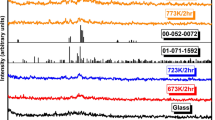
Figure 1 shows the x-ray diffraction (XRD) patterns of 60V2O5-xP2O5-(40 − x)Bi2O3 systems of the synthesized samples. The XRD patterns confirm the amorphous features of the samples under test. It can be observed from the XRD studies that homogenous glasses are formed for P2O5 concentrations between 0 and 20 mol%. The XRD patterns (Fig. 1) indicate a glassy behavior with a broad hump at 2θ = 20–25° with no indication of diffraction peaks. Figure 1b shows the pattern of 60V2O5-15P2O5-25Bi2O3 glass–ceramics nanocrystals which contains a number of peaks that were determined after its annealing at crystallization temperature Tc = 280°C, estimated from differential scanning calometry (DSC) analysis (Fig. 2). Figure 1b shows that, by adjusting the temperature of annealing, one can control the amount of nanocrystalline grains formed in the sample under investigation, and hence its effect on electrical conductivity (see Fig. 5, below). We used Sherrer’s equation,13 to calculate the average particle sizes, D, of the precipitated nanocrystals.
where λ (nm) represents the wavelength of CuKα radiation, β represents the corrected full width at half maximum of the diffraction peak in radians, and k~1, θ is the Bragg angle of the XRD peak. D was found to be about 22 ± 2 nm for the sample under study, and the calculated particle size is the average as obtained by applying Sherrer’s equation on all Bragg’s equation shows the corresponding V2O5 found in the XRD pattern (Fig. 1b).
Differential Scanning Calometry (DSC)
Figure 2 shows DSC thermogram of 60V2O5-15P2O5-25Bi2O3 glass that exhibits an exothermic peak corresponding to crystallization (Tc) and an endothermic dip due to glass transformation (Tg). It is shown that the viscosity and thermal stability of the glass depend on the temperature difference ΔT = Tc − Tg of the glasses.14,15,16,17,18,19 On the other hand, these glasses have exhibited that the difference ΔT = 70°C is quite large and that many shapes such as sheets and disks can be prepared from these glasses. Obviously, the studies of DSC on many glasses showed that glass transformation (Tg) depends on the coordination number of the network former and the construction of non-bridging oxygen (NBO) atoms.20 Also, the construction of NBO causes a decrease in the Tg but increases when the coordination number of the network former increases.20
Electrical Conductivity
Figure 3 shows the dc electrical conductivity of the prepared glass samples that were measured in the temperature range of 300–500 K. The conductivity can be analyzed by the following equation:21:
where σ0 is the pre-exponential factor and W is the activation energy for conduction. The conductivity data may be fitted to straight lines and the activation energy and the pre-exponential factor were determined.
Figure 3 shows the temperature dependence of dc conductivity (σ) for the different glass compositions. It can be seen that the conductivity increases smoothly with increasing temperature. The logarithmic conductivity in the temperature range, 300–500 K, exhibits a linear dependence on the reciprocal temperature, as shown in Fig. 3. The conductivity decreases with increasing P2O5 content (Fig. 3), indicating that dc conductivity dependence of the composition. The activation energy is calculated from the slope of the plots (Fig. 3) and shown in Fig. 4 in the range of 0.23–0.31eV.
The activation energy increases with increasing P2O5, which is observed from Fig. 4. Obviously, as the concentration of P2O5 increases, the electrical conductivity decreases, and the activation of electrical content conduction increases. The low value of electrical conductivity and high value of activation energy are similar to those of V2O5- P2O5 glasses.3 By increasing the P2O5 content, the conductivity and activation energy may change, and, hence, help to detect the structural changes as a consequence of the content and the decrease of the Bi2O3 oxide content. On the other hand, the addition of P2O5 in vanadate glasses decreases the conductivity as a result of decreasing the NBOs by replacing a covalent bond with an ionic bond.3 The NBOs clearly play a defining role in the glass properties. It is obvious from Fig. 4 that the magnitude of activation energy is higher for those compositions which have lower conductivity.
This result is consistent with electronic behavior.12 The conduction process is believed to occur by electron hopping between the ions existing in different valence states in the glass. The dc conductivity in Fig. 3 varied linearly versus 1000/T, indicating that the conduction of the system under investigation is mainly electronic conduction (Fig. 5).13
Figure 6 presents the variation in conductivity with P2O5 content for different glass–ceramic nanocrystals. It can be seen that these corresponding glass–ceramic nanocrystals show high conductivity compared to the samples under test in the glassy phase.14,22,23 The conductivity of glass–ceramic nanocrystals is increased due to the presence of nanocrystals with an average grain size of 22 nm as estimated in the XRD results. Such increases in conductivity have been reported in other studied SrTiO3–PbO2–V2O5 glasses.2 With an increase in conductivity by nanocrystallization, the activation energies for conduction were found to be Wnano = 0.19–0.23 eV at high temperatures which are much lower than those for the as-received glasses Wglass = 0.23–0.31 eV at high temperatures. In the V2O5–P2O5–Bi2O3 system of the present investigation, the P2O5 addition decreased the conductivity (Fig. 6) as a result of decreasing NBO24 which is due to moving the charge carriers with lower mobility. Obviously, in the initial glass, there is a random distribution of charges that is improved by annealing. The annealing at the crystallization temperature leads to the formation of nanocrystallites of V2O5 implanted in the glass structure. The average grain size of these nanocrystals is small, about 22 nm, as reported in XRD, hence the improvement of the electrical conductivity of glass–ceramic nanocrystal systems under study (Fig. 7).
Conclusions
Glasses in the xP2O5-(40 − x)Bi2O3-60V2O5 system are fabricated by conventional melt-quenching technique. By annealing glasses at the crystallization temperature, TC obtained from DSC, they were transformed into glass–ceramic nanocrystals. The average grain size of 22-nm nanocrystals was implanted in the glass structure. The electrical properties activation energy increases with increasing P2O5 content. The resulting xP2O5-(40-x)Bi2O3-60V2O5 glass and glass–ceramic crystals, with 0 ≤ x ≤ 20 (mol%), have been studied. It was observed that the dc conductivity decreases and nanocrystals show much higher electrical conductivity than the initial glasses. The conduction in the present glass system and glass–ceramics were confirmed to be a result of electronic conduction.
Data Availability
The data and materials are available in the manuscript.
References
A.M. Al-syadi, Y. El Sayed, M.M. El-Desoky, and M.S. Al-Assiri, Solid State Sci. 26, 72–82 (2013).
M.M. El-Desoky, H.S.S. Zayed, F.A. Ibrahim, and H.S. Ragab, Physica B 404, 4125–4131 (2009).
R.V. Barde, and S.A. Waghuley, J. Phys. Conf. Ser. 365, 012019 (2012).
D. Sreenivasu, and V. Chandramouli, Bull. Mater. Sci. 23, 281 (2000).
D.K. Shukla, and S. Mollah, Indian J. Pure Appl. Phys. 45, 52 (2007).
A. Al-Hajry, A.A. Soliman, and M.M. El-Desoky, Thermochimica Acta 427, 181 (2005).
J. Simockov, P. Miklos, and V. Saly, Acta Physica Slovaca 50, 685 (2000).
A.M. Al-Shukri, G.D. Khattak, and M.A. Salim, J. Mater. Sci. 35, 123 (2000).
M.G. Moustafa, J. Ceram. Int. 42, 17723–17730 (2016).
R.V. Barde, and S.A. Waghuley, J. Adv. Ceram. 2, 246–251 (2013).
T. Sujatha, T. Sankarappa, J.S. Ashwajeet, R. Ramanna, and S.M. Hanagodimath, J. Adv. Chem. Sci. 1, 157–159 (2015).
R.V. Barde, and S.A. Waghuley, J. Adv. Ceram. 2, 246–251 (2013).
J. Lou, and N. Audebrand, Adv. x-ray Anal. 41, 556 (1997).
M.S. Al-Assiri, S.A. Salem, and M.M. El-Desoky, J. Phys. Chem. Solids 67, 1873 (2006).
M.M. El-Desoky, and I. Kashif, Phys. Stat. Sol. (a) 194, 89 (2002).
M.M. El-Desoky, J. Mater. Sci. Mater. Electron. 14, 215 (2003).
A. Al-Shahrani, A. Al-Hajry, and M.M. El-Desoky, Phys. Stat. Sol. (a) 300, 378 (2003).
H. Moi, T. Kitami, H. Sakata, and J. Non-Cryst, Solids 168, 157 (1994).
M.M. El-Desoky, N.M. Tashtoush, and M.H. Habib, J. Mater. Sci. Mater. Electron. 16, 533 (2005).
M.M. El-Desoky, and M.S. Al-Assiri, Mater. Sci. Eng. B 37, 237 (2007).
N.F. Mott, and J. Non-Cryst, Solids 1, 1 (1968).
J.E. Garbarczyk, P. Jozwiak, M. Wasiucionek, and J.L. Nowinski, Solid State Ionics 175, 691 (2004).
J.E. Garbarczyk, P. Jozwiak, M. Wasiucionek, and J.L. Nowinski, Solid State Ionics 177, 2585 (2006).
J.E. Garbarczyk, P. Jozwiak, M. Wasiucionek, and J.L. Nowinski, J. Power Sources 173, 743 (2007).
T.K. Pietrzak, M. Wasiucionek, and J.E. Garbarczyk, Nanomaterials 11, 1321 (2021).
L. Yu-Ting, Z. Jing-Jing, W. Zhi-Qiang, X. Fei-Long, and L. Hai, Mater. Sci. Poland 38, 28–33 (2020).
N.S. Vedeanu, I.B. Cozar, R. Stanescu, R. Stefan, D. Vodnar, and O. Cozar, Bull. Mater. Sci. 39, 697–702 (2016).
A.A. Raskovalova, N.S. Saetovab, and I.S. Popovc, Chim. Technol Acta 8, 190 (2021).
M. Laourayed, M. Elmoudane, A. Guenbour, M. Tabyaoui, A. Bellaouchou, A. Ghanimi, and A. Sabbar, J. Mater. Environ. Sci. 8, 2932–2939 (2017).
C. Courtney, and W. Lothar, J. Non-Crystall. Solids 8, 196 (2020).
Acknowledgments
The authors wish to express their gratitude to Professor Dr., M.M.Desoky, Physics Department – Faculty of Science – Seuz University – Suez – Egypt, to review research scientifically.
Funding
There is no funding statement for this manuscript.
Author information
Authors and Affiliations
Contributions
Author: F. A. Ibrahim. I have Conceived and designed the analysis. I have Collected the data. I have Contributed data or analysis tools. I have Performed the analysis of all measurements. I have Wrote the paper from first to final. I have submitted this paper to Silicon Journal.
Corresponding author
Ethics declarations
Conflict of interest
There is no any possible conflict of interest for this manuscript from any side or authority.
Consent to Participate
The author participate in this manuscript.
Consent for Publication
The author which publication this manuscript in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, F.A. Electrical Conductivity Enhancement of V2O5-P2O5-Bi2O3 Glasses by Nanocrystallization. J. Electron. Mater. 51, 621–625 (2022). https://doi.org/10.1007/s11664-021-09308-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09308-6