Abstract
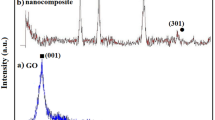
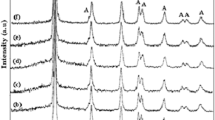
N,F-TiO2 and SiO2 nanoparticles have been synthesized by a sol–gel method and grown on reduced graphene oxide by a solvothermal method at different molar ratios. The microstructure and morphology of the N,F-TiO2/SiO2/rGO nanocomposite were investigated by Fourier-transform infrared spectroscopy, x-ray diffraction analysis, field-emission scanning electron microscopy, transmission electron microscopy, energy-dispersive x-ray spectroscopy, and Brunauer–Emmett–Teller surface area (SBET) measurements. The synthesized nanocomposite was used for photocatalytic degradation of Methyl Red (MR) dye. Ultraviolet–visible (UV–Vis) spectrophotometry was used to determine the degree of dye degradation before and after contact with the nanocomposite, and the absorbance was measured at 518 nm. The results confirmed that the N,F-TiO2/SiO2/rGO nanocomposite degraded 95% of MR after 60 min under visible-light irradiation. Factors affecting its photocatalytic ability were investigated and optimized. The results showed that the highest degradation efficiency was observed when 1.5 mL silica sol was used to synthesize the nanocomposite. Finally, the mechanism of Methyl Red degradation was investigated.
Similar content being viewed by others
References
S. Benkhaya, S. El Harfi, and A. El Harfi, Appl. J. Environ. Eng. Sci. 3, 311 (2017).
S. Arivoli and M. Hema, Int. J. Phys. Sci. 2, 10 (2007).
M.A. Mohammed, A. Shitu, and A. Ibrahim, Res. J. Chem. Sci. 4, 91 (2014).
C. Zhang, Z. Zhu, H. Zhang, and Z. Hu, J. Environ. Sci. 24, 1021 (2012).
Y. Wang, B. Gao, Q. Yue, and Y. Wang, J. Environ. Sci. 23, 1626 (2011).
A. Ayati, M. Niknam Shahrak, B. Tanhaei, and M. Sillanpää, Chemosphere 160, 30 (2016).
C. Sahoo, A.K. Gupta, and A. Pal, Desalination 181, 91 (2005).
M. Ghaedi, A. Najibi, H. Hossainian, A. Shokrollahi, and M. Soylak, Toxicol. Environ. Chem. 94, 40 (2012).
Y. Badr, M. Abd El-Wahed, and M. Mahmoud, J. Hazard. Mater. 154, 245 (2008).
H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard, and J.M. Herrmann, Appl. Catal. B 39, 75 (2002).
D. Sponza and M. Işik, Enzyme Microb. Technol. 31, 102 (2002).
P.V. Nidheesh, M. Zhou, and M.A. Oturan, Chemosphere 197, 210 (2018).
D. Georgiou, A. Aivazidis, J. Hatiras, and K. Gimouhopoulos, Water Res. 37, 2248 (2003).
S. Natarajan, H.C. Bajaj, and R.J. Tayade, J. Environ. Sci. 65, 201 (2018).
G. McMullan, C. Meehan, A. Conneely, N. Kirby, T. Robinson, P. Nigam, I.M. Banat, R. Merchant, and W.F. Smyth, Appl. Microbiol. Biotechnol. 56, 81 (2001).
W. Lu, T. Xu, Y. Wang, H. Hu, N. Li, X. Jiang, and W. Chen, Appl. Catal. B-Environ. 180, 20 (2016).
M. Eghbali-Arani, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, F. Ahmadi, and S. Pourmasoud, Ultrason. Sonochem. 43, 120 (2018).
J. Madhavan, P. Maruthamuthu, S. Murugesan, and S. Anandan, Appl. Catal. B-Environ. 83, 8 (2008).
S.P. Albu, A. Ghicov, S. Aldabergenova, P. Drechsel, D. LeClere, G.E. Thompson, J.M. Macak, and P. Schmuki, Adv. Mater. 20, 4135 (2008).
Y.H. Yu, Y.P. Chen, and Z. Cheng, Int. J. Hydrog. Energy 40, 15994 (2015).
X. Hao, Z. Jin, J. Xu, S. Min, and G. Lu, Superlattice Microstruct. 94, 237 (2016).
J. Zhang, Y. Kusumawati, and T. Pauporté, Electrochim. Acta 201, 125 (2016).
J. Du, X. Lai, N. Yang, J. Zhai, D. Kisailus, F. Su, D. Wang, and L. Jiang, ACS Nano 5, 590 (2010).
F. Liu, X. Yan, X. Chen, L. Tian, Q. Xia, and X. Chen, Catal. Today 264, 243 (2016).
T. Xu, L. Zhang, H. Cheng, and Y. Zhu, Appl. Catal. B 101, 382 (2011).
W. Fan, Q. Lai, Q. Zhang, and Y. Wang, J. Phys. Chem. C 115, 10694 (2011).
K. Osako, K. Matsuzaki, T. Susaki, S. Ueda, G. Yin, A. Yamaguchi, H. Hosono, and M. Miyauchi, Chem. Catal. Chem. 10, 3666 (2018).
K.C. Nguyen, M.P. Ngoc, and M.V. Nguyen, Mater. Lett. 165, 247 (2016).
J. Yu, T. Ma, G. Liu, and B. Cheng, Dalton Trans. 40, 6635 (2011).
F. Wang and K. Zhang, Curr. Appl. Phys. 12, 346 (2012).
D. Li, M.B. Müller, S. Gilje, R.B. Kaner, and G.G. Wallace, Nat. Nanotechnol. 3, 101 (2008).
I.V. Lightcap, T.H. Kosel, and P.V. Kamat, Nano Lett. 10, 577 (2010).
G. Williams, B. Seger, and P.V. Kamat, ACS Nano 2, 1487 (2008).
B. Tang and G. Hu, Chem. Vap. Depos. 20, 14 (2014).
T. Bo, W. Zhengwei, W. Huang, L. Sen, M. Tingting, Y. Haogang, and L. Xufei, Nanoscale Res. Lett. 12, 527 (2017).
H.M. Yadav and J.S. Kim, J. Alloys Compd. 688, 123 (2016).
Q. Xiang, J. Yu, and M. Jaroniec, Chem. Soc. Rev. 41, 782 (2012).
Q. Xiang, J. Yu, and M. Jaroniec, J. Am. Chem. Soc. 134, 6575 (2012).
A. Nikokavoura and C. Trapalis, Appl. Surf. Sci. 430, 18 (2018).
X. Li, R. Shen, S. Ma, X. Chen, and J. Xie, Appl. Surf. Sci. 430, 53 (2018).
J. Cha, M. Cui, M. Jang, S.H. Cho, D.H. Moon, and J. Khim, Environ. Geochem. Health 33, 81 (2011).
L. Staudenmaier, Ber. Dtsch. Chem. Ges. 31, 1481 (1898).
H. Ijadpanah-Saravi, M. Zolfaghari, A. Khodadadi, and P. Drogui, Desalin. Water Treat. 57, 14647 (2016).
A. Jonidi Jafari, R. Rezaei Kalantary, A. Esrafili, and H. Arfaeinia, Process. Saf. Environ. 116, 377 (2018).
M. Riazian, J. Nanostruct. 4, 433 (2014).
J. Gao, W. Li, X. Zhao, L. Wang, and N. Pan, Text. Res. J. 89, 517 (2019).
S. Brunauer, P.H. Emmett, and E. Teller, J. Am. Chem. Soc. 60, 309 (1938).
C. Sahoo, A.K. Gupta, and A. Pal, Desalination 181, 91 (2005).
P.P. Hankare, R.P. Patil, A.V. Jadhav, K.M. Garadkar, and R. Sasikala, Appl. Catal. B-Environ. 107, 333 (2011).
T. Welderfael, O.P. Yadav, A.M. Taddesse, and J. Kaushal, Bull. Chem. Soc. Ethiopia 27, 221 (2013).
Y.M. Hunge, V.S. Mohite, S.S. Kumbhar, K.Y. Rajpure, A.V. Moholkar, and C.H. Bhosale, J. Mater. Sci.: Mater. Electron. 26, 8404 (2015).
B.M. Vinoda, M. Vinuth, Y.D. Bodke, and J. Manjanna, J. Environ. Anal. Toxicol. 5, 2161 (2015).
A.D. Vishwanath, S.S. Jadhav, N.M. Eknath, A.E. Athare, and N.H. Kolhe, Orient. J. Chem. 33, 104 (2017).
Y. Wan, J. Chen, J. Zhan, and Y. Ma, J. Environ. Chem. Eng. 6, 6079 (2018).
O.L. Omotunde, A.E. Okoronkwo, A.F. Aiyesanmi, and E. Gurgur, J. Photochem. Photobiol. A 365, 145 (2018).
S.M. Patil, S.P. Deshmukh, K.V. More, V.B. Shevale, S.B. Mullani, A.G. Dhodamani, and S.D. Delekar, Mater. Chem. Phys. 225, 247 (2019).
Acknowledgment
The authors are highly grateful to the Laboratory Complex of Science and Research Branch, I.A.U. for valuable methodological support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samadi, S., Khalili, E. & Allahgholi Ghasri, M.R. Degradation of Methyl Red under Visible Light Using N,F-TiO2/SiO2/rGO Nanocomposite. J. Electron. Mater. 48, 7836–7845 (2019). https://doi.org/10.1007/s11664-019-07585-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07585-w