Abstract
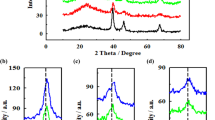
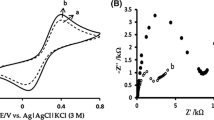
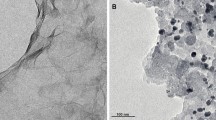
Ni/ZnO with different molar ratios were synthesized by a simple hydrothermal method and used as substrate in the reaction layer of gas diffusion electrodes for a platinum electrodeposition electrocatalyst. Cyclic voltammetry technique was used for platinum electrodeposition on prepared substrate. The physicochemical characterization of the optimized electrocatalyst was done by scanning electron microscopy, x-ray diffraction, and energy-dispersive x-ray. The electrochemical characterization of the platinum electrodeposited on modified carbon substrate with Ni/ZnO particles were studied at a range of Ni concentration in substrate of electrocatalyst by using cyclic voltammetry, linear sweep voltammetry, and electrochemical impedance spectroscopy. The results show that the Ni has a significant effect on performance of prepared electrocatalyst for methanol oxidation reactions (MOR). The presence of 50 wt.% Ni into ZnO in modified carbon substrate shows the good distribution of platinum nanoparticles on the substrate, which generates more active sites for MOR. In addition, the electrochemical surface area of this electrocatalyst reached 105.6 m2 g−1, which was higher than that of commercial Pt/C electrocatalyst (32.45 m2 g−1). The impact of these factors leads to high catalytic activity for MOR. Since methanol can be used as fuel in future portable fuel cells, the synthesized electrocatalysts can provide good conditions for methanol oxidation reactions in these systems.
Similar content being viewed by others
References
X. Lu, W. Wang, Z. Deng, H. Zhu, S. Wei, S.-P. Ng, W. Guo, and C.-M.L. Wu, RSC Adv. 6, 1729 (2016).
H. Kunitomo, H. Ishitobi, and N. Nakagawa, J. Power Sour. 297, 400 (2015).
Y. Cheng, P.K. Shen, and S.P. Jiang, Int. J. Hydrog. Energy 41, 1935 (2016).
A.A. Siller-Ceniceros, M.E. Sánchez-Castro, D. Morales-Acosta, J.R. Torres-Lubian, E. Martínez, and F.J. Rodríguez-Varela, Appl. Catal B: Environ. 209, 455 (2017).
T. Saida, N. Ogiwara, Y. Takasu, and W. Sugimoto, J. Phys. Chem. C 114, 13390 (2010).
R. Chang, L. Zheng, C. Wang, D. Yang, G. Zhang, and S. Sun, Appl. Catal. B: Environ. 211, 205 (2017).
X. Li, H. Wang, H. Yu, Z. Liu, H. Wang, and F. Peng, Electrochim. Acta 185, 178 (2015).
J. Hosseini, M. Abdolmaleki, H.R. Pouretedal, and M.H. Keshavarz, Chin. J. Catal. 36, 1029 (2015).
X. Chen, C. Si, Y. Gao, J. Frenzel, J. Sun, G. Eggeler, and Z. Zhang, J. Power Sour. 273, 324 (2015).
J. Ju, X. Chen, Y. Shi, D. Wu, and P. Hua, J. Ind. Eng. Chem. 20, 1223 (2014).
O. Akyıldırım, H. Yüksek, H. Saral, İ. Ermiş, T. Eren, and M.L. Yola, J. Mater. Sci.: Mater. Electron. 27, 8559 (2016).
S.N. Ab Malek and Y. Mohd, Int. J. Electrochem. Sci. 12, 1561 (2017).
A.M. El-Sawy, H. Tasnim, A.G. Meguerdichian, J. Jin, J.P. Dubrosky, and S.L. Suib, Inorg. Chem. 57, 9977 (2018).
J. Pfrommer, M. Lublow, A. Azarpira, C. Gobel, M. Lucke, A. Steigert, M. Pogrzeba, P.W. Menezes, A. Fischer, T. Schedel-Niedrig, and M. Driess, Angew. Chem. Int. Ed. 53, 5183 (2014).
J. Liu, B. Chen, Z. Ni, Y. Deng, X. Han, W. Hu, and C. Zhong, Chem. Electro. Chem. 3, 537 (2016).
M. Nischk, P. Mazierski, Z. Wei, K. Siuzdak, N.A. Kouame, E. Kowalska, H. Remita, and A. Zaleska-Medynsk, Appl. Surf. Sci. 387, 89 (2016).
T. Takashima, T. Sano, and H. Irie, Electrochemistry 84, 784 (2016).
K. Zhang, W. Yang, C. Ma, Y. Wang, C. Sun, Y. Chen, P. Duchesne, J. Zhou, J. Wang, Y. Hu, M.N. Banis, P. Zhang, F. Li, J. Li, and L. Chen, NPG Asia Mater. 7, e153 (2015).
T.-Y. Yung, J.-Y. Lee, and L.-K. Liu, Sci. Technol. Adv. Mater. 14, 03500 (2013).
H. Wang, X. Wang, J. Zheng, F. Peng, and H. Yu, Chin. J. Catal. 35, 1687 (2014).
C.S. Sharma, A.S.K. Sinha, and R.N. Singh, Int. J. Hydrog. Energy 39, 20151 (2014).
S. Trasatti and O.A. Petrii, J. Electroanal. Chem. 327, 353 (1992).
P.K. Shen, C. Xu, R. Zeng, and Y. Liu, Electrochem. Solid-State Lett. 9, A39 (2006).
A. Banisharif, S. Hakim Elahi, A. Anaraki Firooz, A.A. Khodadadi, and Y. Mortazavi, Int. J. Nanosci. Nanotechnol. 9, 193 (2013).
Acknowledgments
The authors would like to acknowledge the support of the Fuel Cell Research Laboratory (FCRL) of Shahid Rajaee Teacher Training University (Tehran, Iran).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdullah Mirzaie, R., Anaraki Firooz, A. & Bakhtiari, M. Highly Efficient Electrocatalyst of Pt Electrodeposited on Modified Carbon Substrate with Ni/ZnO for Methanol Oxidation Reaction. J. Electron. Mater. 48, 2971–2977 (2019). https://doi.org/10.1007/s11664-019-07054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07054-4