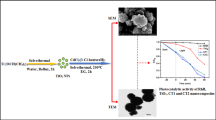
Abstract
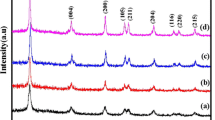
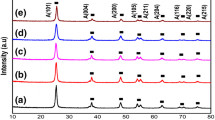
An economical chemical route is used to synthesize a titanium oxide-based nanocomposite (NC) which has properties that may help to develop photocatalysts with improved efficiency in the visible region of solar light. Investigation of opto-electronic properties has been carried out for a CdS, TiO2 and CdS-TiO2 NC at room temperature. Structural properties of the CdS and TiO2 nanoparticles and the CdS-TiO2 NC were investigated using x-ray diffraction and transmission electron microscopy techniques. How incorporation of CdS nanoparticles affects the structural properties of pure TiO2 was also studied. Change in optical behavior was compared using UV–visible and photoluminescence spectra, and the presence of residual functional groups were indicated by Fourier-transform infrared analysis of the samples. The measurement of band levels was carried out by cyclic voltammetry (CV) and the band gap estimated from UV–visible was compared to that obtained by CV. The electron paramagnetic resonance technique was used for the investigation of the electronic properties of the samples.
Similar content being viewed by others
References
N. Zhang, S. Liu, X. Fu, and Y.J. Xu, J. Mater. Chem. 22, 5042 (2012).
P.H.C. Camargo, K.G. Satyanarayana, and F. Wypych, Mater. Res. 12, 11 (2009).
L. Cao, X. Wu, Q. Wang, and J. Wang, J. Photochem. Photobiol. B 178, 440 (2018).
M. Salarian, W.Z. Xu, Z. Wang, T.-K. Sham, and P.A. Charpentier, ACS Appl. Mater. Interfaces 6, 16918 (2014).
E. Bet-moushoul, Y. Mansourpanah, Kh Farhadi, and M. Tabatabaei, Chem. Eng. J. 283, 29 (2016).
K. Zhang and H.J. Choi, Materials 7, 3399 (2014).
A. Truppi, F. Petronella, T. Placido, M. Striccoli, A. Agostiano, M.L. Curri, and R. Comparelli, Catalysts 7, 100 (2017).
D. Zhao and C.-F. Yang, Renew. Sustain. Energy Rev. 54, 1048 (2016).
G.-S. Li, D.-Q. Zhang, and J.C. Yu, Environ. Sci. Technol. 43, 709 (2009).
S. Bera, S.B. Rawal, H.J. Kim, and W.I. Lee, ACS Appl. Mater. Interfaces 6, 9654 (2014).
H. He, A. Chen, H. Lv, and C. Li, Mod. Phys. Lett. B 27, 1341015 (2013).
S. Chaguetmi, F. Mammeri, M. Pasut, S. Nowak, H. Lecoq, P. Decorse, C. Costentin, S. Achour, and S. Ammar, J. Nanopart. Res. 15, 2140 (2013).
T. Jafari, E. Moharreri, A.S. Amin, R. Miao, W. Song, and S.L. Suib, Molecules 21, 900 (2016).
W. Aashir, Z. Qianpeng, T.M. Mahdi, L.S. Fung, G. Leilei, H. Jin, M. Xiaoliang, and F. Zhiyong, Sci. Bull. 61, 86 (2016).
J.C. Védrine, Catalysts 7, 341 (2017).
W.-W. So, K.-J. Kim, and S.-J. Moon, Int. J. Hydrog. Energy 29, 229 (2004).
C.-R. Ke, J.-S. Guo, Y.-H. Su, and J.-M. Ting, Nanotechnology 27, 435405 (2016).
P.D. Tran, L.H. Wong, J. Barber, and J.S. Loo, Environ. Sci. 5, 5902 (2012).
L.-L. Tan, W.-J. Ong, S.-P. Chai, and A.R. Mohamed, Nanoscale Res. Lett. 8, 465 (2013).
Z. Chen, S. Liu, M.-Q. Yang, and Y.-J. Xu, ACS Appl. Mater. Interfaces 5, 4309 (2013).
K.-H. Lin, C.-Y. Chuang, Y.-Y. Lee, F.-C. Li, Y.-M. Chang, I.-P. Liu, S.-C. Chou, and Y.-L. Lee, J. Phys. Chem. C 116, 1550 (2012).
G.-S. Li, D.-Q. Zhang, and J.C. Yu, Environ. Sci. Technol. 43, 7079 (2009).
D. Wu, F. Wang, Y. Tana, and C. Li, RSC Adv. 6, 73522 (2016).
T. Senasu and S. Nanan, J. Mater. Sci. Mater. Electron. 28, 17421 (2017).
H. Zhang, G. Chen, and D.W. Bahnemann, J. Mater. Chem. 19, 5089 (2009).
L.J. Diguna, Q. Shen, J. Kobayashi, and T. Toyoda, Appl. Phys. Lett. 91, 023116 (2007).
A.-M. Ilyas, M.A. Gondal, Z.H. Yamani, and U. Baig, Int. J. Energy Res. 41, 1422 (2017).
M.M. Khan, S.A. Ansari, D. Pradhan, M. Omaish Ansari, D.H. Han, J. Leea, and M.H. Cho, J. Mater. Chem. A 2, 637 (2014).
S.A. Ansari and M.H. Cho, Sci. Rep. 6, 25405 (2016).
Y. Bessekhouad, D. Robert, and J. Weber, J. Photochem. Photobiol. A 163, 569 (2004).
W. Zhu, X. Liu, H. Liu, D. Tong, J. Yang, and J. Peng, J. Am. Chem. Soc. 132, 12619 (2010).
S. Liu, N. Zhang, Z.-R. Tang, and Y.-J. Xu, ACS Appl. Mater. Interfaces 4, 6378 (2012).
N. Zhang, S. Liu, X. Fu, and Y.-J. Xu, J. Mater. Chem. 22, 5042 (2012).
Z. Chen and Y.-J. Xu, ACS Appl. Mater. Interfaces 5, 13353 (2013).
W. Donga, F. Pana, L. Xua, M. Zhengc, C.H. Sowc, K. Wub, G.Q. Xua, and W. Chena, Appl. Surf. Sci. 349, 279 (2015).
G. Xu, S. Ji, C. Miao, G. Liu, and C. Ye, J. Mater. Chem. 22, 4890 (2012).
S.K. Haram, B.M. Quinn, and A.J. Bard, J. Am. Chem. Soc. 123, 8860 (2001).
P. Prasannalakshmi, N. Shanmugam, and A.S. Kumar, J. Appl. Electrochem. 47, 889 (2017).
L.-B. Xiong, J.-L. Li, B. Yang, and Y. Yu, J. Nanomater. 13, 831524 (2012).
T.A. Konovalova, L.D. Kispert, and V.V. Konovalov, J. Phys. Chem. B 103, 4672 (1999).
L. Wu, J.C. Yu, and X. Fu, J. Mol. Catal. A Chem. 244, 25 (2006).
S. Yu, J. Hu, and J. Li, Int. J. Photoenergy (2014). https:// doi.org/10.1155/2014/854217.
S. Dutta, R. Sahoo, C. Ray, S. Sarkar, J. Jana, Y. Negishib, and T. Pa, Dalton Trans. 44, 193 (2015).
T.K. Jana, A. Pal, and K. Chatterjee, J. Alloys Compd. 583, 510 (2014).
A.K. Gupta and R. Kripal, Spectrochim. Acta Part A 96, 626 (2012).
R. Kripal and U.M. Tripathi, Adv. Sci. Eng. Med. 9, 130 (2017).
B.S. Rao, B.R. Kumar, V.R. Reddy, and T.S. Rao, Chalcogenide Lett. 8, 177 (2011).
E. Kucur, J. Riegler, G.A. Urban, and T. Nann, J. Chem. Phys. 119, 2333 (2003).
V. Bala, S.K. Tripathi, and R. Kumar, J. Nanoeng. Nanomanuf. 4, 2157 (2014).
K. Das and S.K. De, J. Phys. Chem. C 113, 3494 (2009).
P. Praveen, G. Viruthagiri, S. Mugundan, and N. Shanmugam, Spectrochim. Acta Part A 120, 548 (2014).
J. Yang, J.-H. Zeng, S.-H. Yu, L. Yang, G.-E. Zhou, and Y.-T. Qian, Chem. Mater. 12, 3259 (2000).
T.F. Yen, Electron Spin Resonance of Metal Complexes (New York: Plenum press, 1969).
A. Lund, M. Shiotani, and S. Shimada, Principles and Applications of ESR Spectroscopy (Berlin: Springer, 2011).
Acknowledgments
The authors are gratified to the Head, SAIF, I. I. T. Mumbai, Powai, Mumbai, for providing the facility of the EPR spectrometer. One of the authors, Garima Vaish, is grateful to the Head, Department of Physics, University of Allahabad, Allahabad, for providing departmental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kripal, R., Vaish, G. & Tripathi, U.M. Comparative Study of EPR and Optical Properties of CdS, TiO2 and CdS-TiO2 Nanocomposite. J. Electron. Mater. 48, 1545–1552 (2019). https://doi.org/10.1007/s11664-018-06894-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-06894-w