Abstract
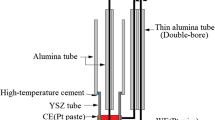
Gibbs energy of formation of NiTiO3 (ilmenite) relative to its component oxides, NiO (rock salt) and TiO2 (rutile), has been measured employing the solid-state electrochemical cell,
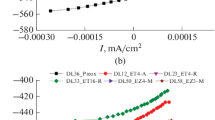
. between 994 and 1371 K. The open-circuit electromotive force (emf) of the preceding solid-state galvanic cell was found to be reversible and to vary linearly as a function of temperature in the range of measurement. The results obtained in this study give for the reaction
. Combining the Gibbs energy of formation of NiTiO3 (ilm) from the component oxides with that for the formation of NiO (rs) from its elements gives for the reaction
. Differential thermal analysis (DTA) of NiTiO3 (ilm) between 373 and 1623 K indicated that NiTiO3 (ilm) undergoes a reversible order-disorder phase transformation between 1540 and 1594 K. Based on the ideal mixing of cations on the cationic sublattice of NiTiO3 (ilm) and a critical phase transformation of 1568 K obtained from the DTA, the Gibbs energy change for the order-disorder phase transformation in NiTiO3 (ilm) is obtained as
.
Similar content being viewed by others
References
T. Armbruster: J. Solid State Chem., 1981, vol. 36, pp. 275–88.
A. Muan: J. Am. Ceram. Soc., 1992, vol. 75 (6), pp. 1357–60.
G. Sankar, C.N.R. Rao, and T. Rayment: J. Mater. Chem., 1991, vol. 1 (2), pp. 299–300.
Y. Takita, T. Ishihara, and M. Hashida: J. Chem. Soc.—Chem. Comm., 1990, vol. 18, pp. 1247–48.
J.M. Santamaria, E.E. Miro, and E.E. Wolf: Ind. Eng. Chem. Res., 1991, vol. 30 (6), pp. 1157–65.
S. Damyanova, A. Spojakina, and K. Jiratova: Appl. Catalysis A-General, 1995, vol. 125 (2), pp. 257–69.
T. Arunarkavalli, G.U. Kulkarni, G. Sankar, and C.N.R. Rao: Catalysis Lett., 1993, vol. 17 (1–2), pp. 29–37.
A. Navrotsky and A. Muan: J. Inorg. Nucl. Chem., 1970, vol. 32, pp. 3471–84.
L.G. Evans and A. Muan: Thermochim. Acta, 1971, vol. 2, pp. 121–34.
R.W. Taylor and H. Schmalzried: J. Phys. Chem., 1964, vol. 68 (9), pp. 2444–49.
G. Chattopadhyay and H. Kleykamp: Z. Metallkd., 1983, vol. 74 (3), pp. 182–87.
L. Pejryd: Acta Chem. Scand. A, 1984, vol. 38, pp. 247–52.
M. Lerch and W. Laqua: Z. Anorg. Allgem. Chem., 1992, vol. 610, pp. 57–63.
O. Kubaschewski: High Temp.—High Pressure, 1972, vol. 4, pp. 1–12.
G.M. Kale and D.J. Fray: Metall. Mater. Trans. B, 1994, vol. 25B, pp. 373–78.
T.N. Rezukhina: Ph.D. Thesis, Moscow State University, Moscow, 1968.
K.T. Jacob, G.M. Kale, and K.P. Abraham: J. Electrochem. Soc., 1992, vol. 139 (2), pp. 517–20.
G.M. Kale and K.T. Jacob: Metall. Trans. B, 1992, vol. 23B, pp. 57–64.
K.T. Jacob and C.B. Alcock: High Temp.—High Pressure, 1975, vol. 7, pp. 433–39.
R.D. Shannon: Acta Cryst., 1976, vol. 32A, pp. 751–67.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kale, G.M. Electrochemical determination of gibbs energy of formation of NiTiO3 (ilmenite). Metall Mater Trans B 29, 31–38 (1998). https://doi.org/10.1007/s11663-998-0004-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-998-0004-3