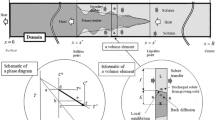
Abstract
An analytical approximate solution to non-linear solute- and heat-transfer equations in the unsteady-state mushy zone of Fe-C plain steel has been obtained, assuming a linear relationship between the solid fraction and the temperature of the mushy zone. The heat transfer equations for both the solid and liquid zone along with the boundary conditions have been linked with the equations to solve the whole equations. The model predictions (e.g., the solidification constants and the effective partition ratio) agree with the generally accepted values and with a separately performed numerical analysis. The solidus temperature predicted by the model is in the intermediate range of the reported formulas. The model and Neuman’s solution are consistent in the low carbon range. A conventional numerical heat analysis (i.e., an equivalent specific heat method using the solidus temperature predicted by the model) is consistent with the model predictions for Fe-C plain steels. The model presented herein simplifies the computations to solve the solute- and heat-transfer simultaneous equations while searching for a solidus temperature as a part of the solution. Thus, this model can reduce the complexity of analyses considering the heat- and solute-transfer phenomena in the mushy zone.








Similar content being viewed by others
Change history
21 September 2018
Running footer on this article appeared incorrectly. It should read 644—VOLUME 49B, APRIL 2018.
References
T. Kawawa, H. Sato, S. Miyahara, T. Koyano, and H.Nemoto: Tetsu-to-Hagane’, 1974, vol. 60, pp. 206-16.
T. Kawawa and H. Tuchida: Tekko-no-Gyouko (Soidification of steel), Appendix 4, ed. By Solidification Comm., Joint Sc. on Iron and Steel Basic Research of ISIJ, ISIJ, Tokyo, 1977.
M. Hirai, K. Kanamaru, and H. Mori: Tekko-no-Gyouko (Soidification of steel), Appendix 4, ed. By Solidification Comm., Joint Sc. on Iron and Steel Basic Research of ISIJ, ISIJ,Tokyo, 1977.
A. Suzuki, T. Suzuki, Y. Nagaoka, and Y. Iwata: J. Jpn. Inst. Met., 1968, vol. 32, pp. 1301-05.
A. Jablonka, K. Harste, and K. Schwerdtfeger: Steel Res., 1991, vol. 62, pp. 24-33.
K. Gryc, B. Smetana, M.Zaludova, K. Michalek, P. Klus, M. Tkadleckova, L. Socha, J. Dobrovska, P. Machovcak, L. Valek, R. Pachlopnik, and B.Chmiel: Mater. Technol., 2013, vol. 47, pp. 569-75.
E. A. Mizikar: Trans. AIME, 1967, vol. 239, pp. 1747-53.
J. Szekely and V. Stanek: Metall.Trans., 1970, vol. 1, pp. 119-26.
J. Matsuno, H. Nakato, and H. Ohi: Tetsu-to-Hagane, 1974, vol. 60, pp. 1023-32.
Y. K. Chaung and K. Schwerdfeger: Arch. Eisenhüttenwes., 1973, vol. 44, pp. 341-7.
P.H. Shingu, K. Takeshita, R. Ozaki, and T. Akiyama: J. Jpn. Inst. Met., 1978, vol. 42, pp. 172-9.
H. D. Brody and M. C. Flemings: Trans. AIME, 1966, vol. 236, pp. 615-24.
T. W. Clyne and W. Kurz: Met. Trans., 1981, vol. 12A, pp. 965-71.
I. Ohnaka and T. Fukusako: Trans. Iron Steel Inst. Jpn., 1977, vol. 17, pp. 410-8.
K. Kumai, A. Sano, T. Ohashi, E. Nomura, and H.Fujii: Tetsu-to-Hagane’, 1974, vol. 7, pp. 894-914.
S. Asai and I. Muchi: Tetsu-to-Hagane’, 1978, vol. 64, pp. 1685-92.
R. N. Hills, D. E. Looper, and P. H. Roberts: Q.J. Mech. Appl. Math. 1983, vol. 36, pp. 505-39.
D. V. Alexandrov: J.Crystal Growth, 2001, vol. 222, pp. 816-21.
K. Takeshita: J.Jpn. Inst.Met., 1983, vol. 47, pp. 647-53.
H. E. Huppert and M. G. Worster: Nature, 1985, vol. 314, pp. 703-7.
D. V. Alexandrov and V. P. Malygin: Int. J. Heat Mass. Transf., 2006, vol. 49, pp. 763-9.
T. Fujimura and J. K. Brimacombe: Trans. Iron Steel Inst. Jpn., 1986, vol. 26, pp. 532-9.
T. Kawawa: Tekko-no-Gyouko (Soidification of steel), Appendix 4, ed. By Solidification Comm., Joint Sc.on Iron and Steel Basic Research of ISIJ, ISIJ, Tokyo, 1977.
H. S. Carslaw and J. G. Jaeger: Conduction of Heat in Solids, 2nd ed., Oxford University Press, 1959, pp. 284.
T. Mitsuo, T. Horii, S. Saito, E. Nomura, Y. Kitamura, and R. Kono: Tetsu-to-Hagane, 1971, vol. 57, pp. 915-41.
M. C. Flemings and G. E. Nero: Trans. Met. Soc. AIME, 1967, vol. 239, pp. 1449-61.
H. Nomura, T. Tarutani, and K. Mori: Tetsu-to-Hagane, 1981, vol. 67, pp. 80-7.
K. Miyazawa: STA, 2001, vol. 2, pp. 59-65.
K. Harste: PhD. Dissertation, Technical University of Clausthal, Clausthal
Y. A. Meng and B. G. Thomas: Metall. Mate. Trans. B, 2003, vol. 34B, pp. 685-705.
A. Hays and Chipman: Trans. Met. Soc. AIME, 1938, vol. 135, pp. 85.
W. A. Fisher, H. Splizer, and M. Hishinuma: Arch. Eisenhüttenwes., 1960, vol. 31, pp. 365.
C. E. Sims: Electric Furnace Steelmaking, vol. 2, John Wiley & Sons, 1962, pp. 99.
W. A. Tiller: J. Iron Steel Inst., 1959, vol. 192, pp. 338-50.
K. Katayama and S. Hattori: Trans. JSME, 1974, vol. 40, pp. 1404-11.
R. A. Buckley and W. Hume-Rothery: J. Iron Steel Inst., 1960, vol. 196, pp. 403-6.
R. E. Grace and G. Derge: Trans. Metall. Soc. AIME, 1958, vol. 212, pp. 33-7.
R.Tanaka: Tetsu-to-Hagane, 1967, vol. 53, pp. 1586-1604.
H. Esaka and S. Ogibayashi: Tetsu-to-Hagane, 1998, vol. 84, pp. 49-54.
D. F. Kalinovich, I. I. Kovenskii, and M. D. Smolin: Izv. Vyssh. Ucheb. Zaved., Fiz., 1971, vol. 9, p. 116.
Acknowledgments
The authors thank Professor K. Ono, University of Kyoto, Japan, for his warm support. They wish to offer thanks to decedent, Professor J.K. Brimacombe, University of British Columbia, Canada, for his support on the development of the base model 30 years ago.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 9, 2017.
Appendices
Appendix A
The error of the approximation of the erf(η) is kept smaller than 0.2 pct for η > 1.73, as shown in Figure 9.
See Appendix Figure 9.
Appendix B
The unknown variables, important physical properties are shown in Table III. The typical carbon partition ratio k in the δ phase (a wide range of values is reported) is used. The left-hand and right-hand sides of Eqs. [71] and [72] are denoted as A, B, and C (B is commonly used). The unknown parameters minimizing the aimed function Z defined as follows were sought: Z = (1 − A/B)2 + (1 − C/B)2
-
(1)
First, the initial values for \(T^{*}\), \(\eta^*\), and \(\eta^{\circ}\) are given. The \(T^{*}\) calculated with the reported formula is used as the initial value for \(T^{*}\). The initial \(\eta^{\circ}\) was 1.0. The \(\eta^*\) was given as \(\eta^* = \gamma\eta^{\circ}\) where the initial γ = 0.3.
-
(2)
Second, the \(\eta^{\circ}\) is changed to minimize Z for the initial \(T^{*}\). Convergence is quickly obtained.
-
(3)
Next, \(\eta^*\) minimizing Z is obtained by changing γ (\(\eta^*=\gamma\eta^{\circ}\) ).
-
(4)
Substituting \(\eta^*\), \(\eta^{\circ}\) obtained by the above process to the left side of [67] provides λ (used in the right side of the Eq. [67], (D: shown above). Substituting this λ into [9], yields the new \(T^{*}\). Using this \(T^{*}\) as the initial \(T^{*}\) and repeating the same process mentioned above [2] to [4] yields the set of (\(T^{*}\), \(\eta^*\), and \(\eta^{\circ}\)) as the three Eqs. [67], [71], and [72] match.
-
(5)
This process is repeated until Z < 0.1, and the four decimal digits do not change in the present analysis.
Appendix C
The carbon concentration in a cylindrical dendrite becomes almost homogeneous at the end of solidification due to the rapid diffusion, as shown in Figure 10.
See Appendix Figure 10.
Rights and permissions
About this article
Cite this article
Fujimura, T., Takeshita, K. & Suzuki, R.O. Mathematical Analysis of the Solidification Behavior of Plain Steel Based on Solute- and Heat-Transfer Equations in the Liquid–Solid Zone. Metall Mater Trans B 49, 644–657 (2018). https://doi.org/10.1007/s11663-017-1149-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1149-8