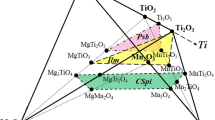
Abstract
The Gibbs free energy of formation of MnV2O4 solid solution saturated with either MnO or V2O3 was experimentally determined at 1823 K (1550 °C) by employing a chemical equilibrium technique. The MnV2O4 solid solution mixed with MnO or V2O3 was brought to equilibrium with either liquid Fe or liquid Cu to measure equilibrium compositions of the liquid metal. Using the available thermodynamic parameters such as Wagner’s interaction parameter for liquid Fe or Gibbs free energy of liquid solution composed of Cu-Mn-V-O, the Gibbs free energy of formation of the MnV2O4 solid solution from constituent oxides (MnO and V2O3) was then determined to be: MnO(s) + V2O3(s) = MnV2O4(MnO-satd.): \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) = −31.4 ± 2 (kJ/mol); MnO(s) + V2O3(s) = MnV2O4(V2O3-satd.): \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) = −37.8 ± 2 (kJ/mol). The \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) values were independent of the oxygen partial pressure within the range from 2.0 × 10−12 to 6.3 × 10−11, employed in the current study.






Similar content being viewed by others
References
S. Mizoguchi and T. Takamura: Proc. 6th Int. Iron and Steel Cong., vol. 1, ISIJ, Tokyo, Japan, 1990, pp. 598–604.
Jung I-H, Kang Y-B, Decterov SA, Pelton AD (2004) . Metall. Mater. Trans. B 35B:259–268
Kang Y-B, Jung I-H, Decterov SA, Pelton AD, Lee H-G (2004) .ISIJ Int., 44:975–983
Kang Y-B, Lee H-G (2004) .ISIJ Int. 44:1006–1015
Kang Y-B, Lee H-G (2005) .ISIJ Int. 45:1543–1551
Kang Y-B, Lee H-G (2005) .ISIJ Int. 45:1552–1560
Kang Y-B, Jung I-H, Lee H-G (2006) . CALPHAD 30:235–247
Kang Y-B, Jung I-H, Lee H-G (2006) .CALPHAD 30:226–234
Kang Y-B, Lee H-G (2010) .ISIJ Int. 50:501–508
Kim M-S, Kang Y-B, Cha W-Y, Lee H-G (2012) .J. Am. Ceram. Soc. 95:2059–2064
R. Kiessling: Non-Metallic Inclusions in Steel, 2nd ed., The Metals Society, U.K., 1978.
Taniguchi T, Satoh N, Saito Y, Kubota K, Kumagai A, Tamura Y, Miki T (2011) .ISIJ Int. 51:1957–1966
M.-S. Kim: M.Sc. Thesis, Pohang University of Science and Technology, Pohang, Korea, 2012.
The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking, Steelmaking Data Sourcebook, revised ed., Gordon and Breach Science Publishers, New York, NY, 1984.
K. Mukai and A. Uchida (1974) .Tetsu-to-Hagané 60:325–336
Sigworth GK, Elliott JF (1974) .Can. Metall. Q. 13:455–461
Bale CW, Chartrand P, Decterov SA, Eriksson G, Hack K, Ben Mahfoud R, Melançon J, Pelton AD, Petersen S (2002) .CALPHAD 26:189–228
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Hack K, Jung I-H, Kang Y-B, Melançon J, Pelton AD, Robelin C, Petersen S (2009) .CALPHAD 33:295–311
Tanahashi M, Furuta N, Taniguchi T (2003) .ISIJ Int. 43:7–13
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980.
Ohtani H, Suda H, Ishida K (1997) .ISIJ Int. 37:207–216
M. Hämäläinen, K. Jääskeläinen, and R. Luoma: CALPHAD, 1990, vol. 14, pp. 125–37.
Hallstedt B, Risold D, Gauckler LJ (1994) .J. Phase Equilib. 15:483–499
Levy A (1964J. Sci. Instrum 41:449–453
Averbukh SM, Smirnov LA, Popel’ SI (1983) .Izv. VUZ Chern. Metall 13:223–224
G.G. Mikhailov and N.N. Lopatko: Russ. Metall., 1997, pp. 49–54.
K.T. Jacob and S.S. Pandit: J. Solid State Chem., 1985, vol. 60, pp. 237–43.
Jacob KT (1977) .J. Electrochem. Soc. 124:1827–1831
Navrotsky A, Kleppa OJ (1967) .J. Inorg. Nucl. Chem 29:2701–2714
E.V. Pannunzio-Miner, J.M. De Paoli, R.D. Sánchez, and R.E. Carbonio: Mater. Res. Bull., 2009, vol. 44, pp. 1586–91.
Dunitz JD, Orgel LE (1957) .J. Phys. Chem. Solids 3:318–323
Acknowledgments
This work was financially supported by POSCO Ltd. through Steel Innovation Program (Project No. 2010Y070) to Graduate Institute of Ferrous Technology, Pohang University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 7, 2013.
Appendix
Appendix
A. Preparation of MnV2O4 Crucibles Saturated by Either MnO or V2O3
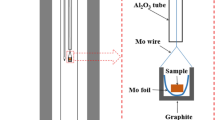
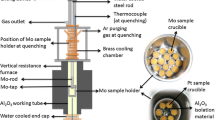
MnO (99.9 pct purity, Kojundo, Chemical Co., Japan) and V2O3 reduced from V2O5 (99.0 pct purity, Daejung Chemicals Ltd., Gyeongg-Do, Korea) by H2 gas in the current study, both in powder form, were mixed to fabricate MnV2O4 crucible saturated by MnO (0.79 : 0.21 in mass ratio) or by V2O3 (0.19 : 0.81 in mass ratio), respectively. Fifty grams of each mixture was compressed into a crucible shape [outer diameter (OD): 30 mm, inner diameter (ID): 20 mm, height (H): 35 mm] by a hydraulic press at pressure around 0.15 GPa as shown in Figure A1. This crucible was put into a MgO holder (OD: 52 mm, ID: 40 mm, H: 70 mm) and placed in the furnace for 24 hours under a H2/CO2 gas mixture (PH 2/PCO 2 = 9) to get MnV2O4 in equilibrium with MnO or V2O3, and to sinter the MnV2O4 crucible as well. After equilibration and sintering, rapid quenching into cold water was followed. An x-ray diffraction (XRD) analysis for each MnV2O4 crucible was conducted to confirm that the MnV2O4 coexisted with MnO or V2O3 as shown in Figure A2.
B. Contamination of MnV2O4 Phase in Contact with the Liquid Fe Under H2/CO2 Gas Mixture
At first, a H2/CO2 gas mixture (PH 2/PCO 2 = 9) was used to control the oxygen partial pressure and to maintain the stable oxide phases (MnO and MnV2O4 saturated with MnO) when liquid Fe was used as the metal solvent. However, there was a considerable Fe dissolution into the oxide phases as shown in Figure B1(a). The proportions of Fe-oxide in MnO and MnV2O4 solid solution were found to be considerably high by EPMA quantitative analysis (Fe:Mn = 1:2 in atomic ratio). Therefore, activity of MnV2O4 spinel phase could not be assumed to be unity. To avoid the contamination by Fe-oxide, purified Ar atmosphere was introduced instead of the H2/CO2 gas mixture, and the EPMA mapping analysis for this sample is shown in Figure B1(b). As can be seen in the figure, Fe contamination into the oxide phases decreased remarkably. An EPMA quantitative analysis indicated that the amount of Fe dissolved into those phases was less than 0.6 mass pct which was considered to be small enough to ignore the effect of Fe-oxide on the activity of MnV2O4 spinel solid solution.
Apart from this, to maintain the H2/CO2 gas mixture condition but to avoid the contamination by oxidation of liquid Fe, the use of liquid Cu in place of Fe was attempted. Although the experimental condition remained the same (MnV2O4 saturated with MnO and H2/CO2 gas condition), the crucible samples after 24 hours-equilibrium experiment with the liquid Cu was subjected to an EPMA mapping analysis for an examination of the oxide phases in the crucible. From Figure B1(c), the oxide phases in equilibrium with the liquid Cu and H2/CO2 atmosphere were not contaminated by Cu-oxide because of the relatively weak oxygen affinity of Cu compared with that of Fe. Therefore, eventually in the current study, two experimental conditions were used to measure the Gibbs free energy of formation of MnV2O4 saturated with either MnO or V2O3:
-
(a)
Liquid Fe + MnV2O4 + purified Ar condition
-
(b)
Liquid Cu + MnV2O4 + H2/CO2 condition
C. Gibbs Free Energy of Formation of Non-stoichiometric MnV2O4
In Section III–B, the \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) values were determined based on Reaction [1], which assumes one mole of stoichiometric MnV2O4 spinel formation from one mole of MnO and V2O3. However, the MnV2O4 phases in equilibrium with liquid metals were not stoichiometric under the experimental conditions of the current study. For nonstoichiometric MnV2O4 formation, Reactions [1], [2], and [11] should be modified as described below:
where x and y are the number of moles of Mn and V participated in non-stochiometric MnV2O4 formation, repectively. Here, the stoichiometric coefficient of dissolved oxygen in Eq. [C2] or that of oxygen gas in Eq. [C3] were determined with the assumption that only divalent Mn and trivalent V are considered to form a nonstoichiometric MnV2O4. From equilibrium composition of MnV2O4 in each sample as summarized in Table III, the stoichiometric coefficients of Mn, V, and O (or oxygen gas) in the above reactions can be derived.
To investigate the effect of nonstoichiometry of MnV2O4 on the Gibbs free energy of formation, \(\Updelta g^\circ_{\rm f}\) of the non-stoichiometric MnV2O4 (\(\Updelta g^\circ_{{\rm f,Mn}_{x} {\rm V}_y {\rm O}_{(x+1.5y)}}, \) hereafter) for Reactions [C2] and [C3] were calculated by considering the following equations:
Then, \(\Updelta g^\circ_{{\rm f,Mn}_{x}{\rm V}_{y}{\rm O}_{({x}+1.5{y})}}\) from constituent oxides (Reaction [C1]) was determined and compared with the values of stoichiometric MnV2O4 as shown in Figure C1. In the figure, \(\Updelta g^\circ_{{\rm f,Mn}_{x}{\rm V}_{y}{\rm O}_{({x}+1.5{y})}}\) and \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) values are close to each other around the stoichiometric MnV2O4 composition, but they show discrepancies as the composition of MnV2O4 moves away from the stoichiometric value. It also indicates that the \(\Updelta g^\circ_{{\rm f,Mn}_{x}{\rm V}_{y}{\rm O}_{({x}+1.5{y})}}\) values are dependent on oxygen partial pressure, which is the opposite of the result in Section III–B. In the current study, however, \(\Updelta g^\circ_{{\rm f, MnV}_2{\rm O}_4}\) is recommended for the following reasons:
-
(a)
In terms of practical application of \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}, \) the reaction of stoichiometric MnV2O4 formation described as Eq. [1] is preferred, rather than Eq. [C1].
-
(b)
Around the stoichiometric MnV2O4 concentration, both \(\Updelta g^\circ_{{\rm f, MnV}_2{\rm O}_4}\) and \(\Updelta g^\circ_{{\rm f,Mn}_{x}{\rm V}_{y}{\rm O}_{({x}+1.5{y})}}\) are similar to each other.
Subsequent investigations are necessary to describe \(\Updelta g^\circ_{{\rm f,MnV}_{2}{\rm O}_{4}}\) on the entire MnV2O4 solid solution range.
D. Enthalpy of Formation Estimated from the Gibbs Free Energy of Formation
The enthalpy of formation of an oxide spinel can be estimated in a simple way by subtracting the entropy contribution from its Gibbs free energy of formation. Jacob[28] suggested an empirical equation of entropy of formation of cubic 2 to 3 spinels from conponent oxides of rock salt and corundum structure:
where \(\Updelta S^{\rm CM}\) is the configurational entropy of the crystal due to cation mixing and \(\Updelta S^{\rm rand}_{\rm J T}\) is the entropy from randomization of the direction of Jahn-Teller distortions. For more details, the readers are invited to the relevant articles by Jacob,[28] and Jacob and Pandit.[27]
The configurational entropy of the oxide spinel could be calculated from cation distribution in tetrahedral and octahedral sublattices. In the case of MnV2O4 spinel structure, cation distribution of normal spinel MnV2O4 at 300 K (27 °C) was proposed as [Mn 2+0.75 V 3+0.75 ]T[Mn 2+0.25 V 3+1.75 ]OO4 from neutron diffraction data by Pannunzio-Miner et al.[30] but the temperature seems to too low to be extrapolated up to 1823 K (1550 °C) relevant to the current study. In the current study, cation distribution of MnV2O4 spinel structure at 1823 K (1550 °C) was calculated using octahedral site preference energies as suggested by Navrotsky and Kleppa.[29]
Generally, cationic configuration of a stoichiometric 2 to 3 oxide spinel AB2O4 is described as:
where λ is the fraction of divalent A ion on the tetrahedral site. In terms of cation interchange between the sublattices, the upper reaction could be also regarded as:
then enthalpy and entropy change of the spinel for the above reaction could be written as follows:
where \( H^{\rm O}_{\hbox{A}^{2+}}\) and \( H^{\rm O}_{\hbox{B}^{3+}}\) are the octahedral site preference energy of A2+ ion and that of B3+ ion, respectively. For entropy change, it is reasonable to take only configurational entropy into account because nonconfigurational contributions to total entropy change were found to be negligible in 2 to 3 oxide spinels.[29] Using the Eq. [D4] and \( H^{\rm O}_{\hbox{Mn}^{2+}}\) and \( H^{\rm O}_{\hbox{V}^{3+}}\) values from the Reference 31, the fraction of Mn2+ on the tetrahedral site of MnV2O4 at 1823 K (1550 °C) was determined to be 0.796, a similar value compared with 0.75.[30] Then, by inserting the fraction of Mn2+ as λ into Eq. [D5] and using Eq. [D1], the entropy of formation and, subsequently, the enthalpy of formation of the MnV2O4 could be obtained.
Rights and permissions
About this article
Cite this article
Kim, MS., Lee, HG. & Kang, YB. Determination of Gibbs Free Energy of Formation of MnV2O4 Solid Solution at 1823 K (1550 °C). Metall Mater Trans B 45, 131–141 (2014). https://doi.org/10.1007/s11663-013-9954-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9954-1