Abstract
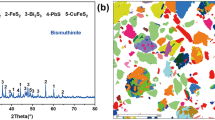
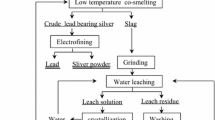
The main purpose of this study is to characterize and separate antimony from a stibnite concentrate through a low-temperature sulfur-fixing smelting process. This article reports on a study conducted on the optimization of process parameters, such as flux and zinc oxide weight percentage, in charging, smelting temperature, smelting duration on the antimony yield, resultant crude antimony grade, and sulfur-fixing rate. A maximum antimony recovery of 97.07 pct, crude antimony grade of 96.45 pct, and 98.61 pct sulfur-fixing rate are obtained when a charge (containing 63.20 wt pct of flux and 21.30 wt pct of stibnite, a flux composition of \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \) = 10/147, where W represents weight, and more than 10 pct of the stoichiometric requirement of zinc oxide dosage) is smelted at 1153 K (880 °C) for 120 minutes. This smelting operation is free from atmospheric pollution because zinc oxide is used as the sulfur-fixing agent. The solid residue is subjected to mineral dressing operation to obtain suspension, which is filtered ultimately to produce a cake, representing the solid particles of zinc sulfide. Based on the results of the chemical content analysis of as-resultant zinc sulfide, more than 90 pct zinc sulfide can be recovered, and the recovered zinc sulfide grade can reach 66.70 pct. This material can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide.

Similar content being viewed by others
References
Z. Yi-feng and Z. Wen-tian: Miner. Process. Extr. Metall., 1984, vol. 3, pp. 687-98.
S. Gui-hua: Trans. Inst. Min. Metall., Sect. C, 1984, vol. 93, pp. 186-92.
T. Lager and K.S.E. Forssberg: Miner. Eng., 1989, vol. 4, pp. 543-56.
T. Mo-tang and J. Gui-zhong: Chin. J. Nonferr. Metal, 2007, vol. 3, pp. 34-36.
Ch. Yong-ming, H. Chao, T. Mo-tang, Y. Wei-yi, T. Chao-bo, and P. Guan-hua: Chin. J. Nonferr. Metal, 2005, vol. 15, pp. 1311–16.
P. Baláă, J. Briančin, V. Šepelák, T. Havlik, and M. Škrobian: Hydrometallurgy, 1992, vol. 31, pp. 201-12.
S. Ubaldini, F. Vegliò, P. Fornari, and C. Abbruzzese: Hydrometallurgy, 2000, vol. 57, pp. 187-99.
W. Jikun and L. Ting: Nonferr. Metal, 2000, vol. 52, pp. 44-8.
L. Lei: YunNan Metall., 2002, vol. 31, pp. 23-25.
P. Baláž and M. Achimovičová: Hydrometallurgy, 2006, vol. 84, pp. 60-68.
Y. Jian-guang, Y. Sheng-hai, and T. Chao-bo: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 523–34.
Acknowledgments
This project was supported by the Non-Ferrious Metals Science Foundation of HNG-CSU. Project (50804056) was supported by the National Nature Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 28, 2010.
Rights and permissions
About this article
Cite this article
Yang, JG., Tang, CB., Chen, YM. et al. Separation of Antimony from a Stibnite Concentrate Through a Low-Temperature Smelting Process to Eliminate SO2 Emission. Metall Mater Trans B 42, 30–36 (2011). https://doi.org/10.1007/s11663-010-9453-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9453-6