Abstract
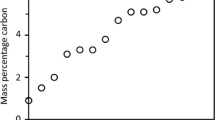
Carbon-dissolution studies were carried out on four coal-chars (ash content ranging from 9.04 to 12.61 wt pct), using the carburizer-cover method, and the rates of carbon transfer into liquid iron at 1550 °C were determined. A theoretical model was developed for estimating the interfacial area of contact between the chars and the liquid iron. Using a force-balance approach, the partial penetration of the particles was calculated numerically and the total solid/liquid contact area was evaluated for a range of system parameters. The wettability was found to have a very significant effect on the area of contact. An improvement in wetting reduced the upward force due to surface tension, thereby increasing the downward penetration of particles in the liquid and the contact area. While two chars showed a monotonic increase in carbon pickup by the liquid iron, a two-stage behavior was observed for the remaining two chars. Stage I, which corresponds to short times of contact, showed a much higher rate of carbon dissolution, as compared to stage II during later times. The slow rate of carbon dissolution in stage II was attributed to high levels of interfacial blockage by reaction products, which resulted in many fewer areas of contact between the carbonaceous material and the liquid iron. First-order dissolution rate constants (×103 ms−1) were computed for stage I in all chars, and the observed trend was as follows: 0.01795 (Char 1)>0.00954 (Char 4)>0.0061 (Char 3)>0.00274 (Char 2). These results compare well with the dissolution rate constants quoted in the literature. Char 1, which had the highest rate constant, also had the lowest concentration of reducible oxides (e.g., silica) among all chars. The consumption of solute carbon through silica reduction could affect the carbon levels in the liquid iron. Due to reduction reactions, the experimentally measured rates of carbon dissolution are expected to be slower than the inherent rates of carbon dissolution into the liquid metal. This study shows strong evidence that chemical reactions at the interface play an important role in determining the rate of char dissolution into liquid metal.
Similar content being viewed by others
References
K. Yamaguchi, H. Ueno, and K. Tamura: Iron Steel Inst. Jpn. Int., 1992, vol. 32, pp. 716–24.
K. Yamaguchi, H. Ueno, and K. Tamura: Tetsu-tu-Hagané, 1992, vol. 78, pp. 1214–21.
C. Yamagata, S. Suyama, S. Horisaka, K. Takatani, Y. Kajiwara, S. Komatsu, H. Shibuta, and Y. Amingaga: Iron Steel Inst. Jpn. Int., 1992, vol. 32, pp. 725–32.
R.J. Haywood, J.S. Truelove, and M.J. McCarthy: 53rd Ironmaking Conf. Proc., Chicago, IL, 1994, pp. 432–42.
R. Khanna and V. Sahajwalla: Phys. Status Solidi B, 1999, vol. 213, pp. 47–58.
A.S. Mehta, A.S. Sahajwalla, and V. Sahajwalla: Iron Steel Inst. Jpn. Int., 2004, vol. 43 (10), pp. 1512–18.
R.G. Olsson, V. Komp, and T.F. Perzak: Trans TMS-AIME, 1966, vol. 236, pp. 426–29.
M. Kosaka and S. Minowa: Trans. Iron Steel Inst. Jpn., 1968, vol. 6 (8), pp. 392–400.
L. Kalvelage, J. Markert, and J. Potschke: Arch. Eisenhuttenwes., 1979, vol. 50 (3), pp. 107–10.
S.O. Ericsson and P.O. Melberg: Scand. J. Metall., 1981, vol. 10, pp. 15–18.
C. Wu: Ph.D. Thesis, The University of New South Wales, Sydney, Australia, 1998.
S. Orsten and F. Oeters: Process Technology Conf. Proc., Washington, DC, 1986, vol. 6 (3), pp. 143–55.
R. Olivares: Ph.D. Thesis, The University of New Castle, New Castle, 1997.
S.O. Ericsson and P.O. Melberg: Scand. J. Metall., 1981, vol. 10 (1), pp. 15–18.
V. Sahajwalla and R. Khanna: Metall. Mater. Trans. B, 2000, vol. 31B, pp. 1517–25.
V. Sahajwalla and R. Khanna: Scand. J. Metall., 2003, vol. 32 (1), pp. 53–57.
S. Orsten and F. Oeters: W.O. Philbrook Memorial Symp., Toronto, 1988, ISS, Warrendale, PA, 1988, pp. 27–38.
C. Wu, R. Wiblen, and V. Sahajwalla: Metall. Mater. Trans. B, 2000, vol. 31B, 1099–1104.
S.T. Cham, V. Sahajwalla, R. Sakurovs, H. Sun, and M. Dubikova: Iron Steel Inst. Jpn. Int., 2004, vol. 44 (11), pp. 1835–41.
H.W. Gudenau, J.P. Mulanza, and D.G.R. Sharma: Steel Res., 1990, vol. 61, pp. 97–104.
M. Peters, H.W. Gudenau, M. Scheiwe, and R. Sieger: 2nd Eur. Ironmaking Congr., Glasgow, Scotland, 1991, Institute of Metals, London, 1991, pp. 205–23.
M.B. Mourao, G.G. Krishna Murthy, and J.F. Elliott: Metall. Trans. B, 1993, vol. 24B, pp. 629–37.
L. Liming, V. Sahajwalla, and D. Harris: Energy Fuels, 2000, vol. 14, pp. 869–76.
L. Liming, V. Sahajwalla, C. Kong, and D. Harris: Carbon, 2000, vol. 39, pp. 1821–33.
Y. Shigeno, M. Tokuda, and M. Ohtani: Trans. Jpn. Inst. Met., 1985, vol. 26 (1), pp. 33–43.
C. Wu and V. Sahajwalla: Metall. Trans. B, 2000, vol. 31B, pp. 243–51.
A. Ganguly and K.J. Reid: Steel Res., 1992, vol. 63 (7), pp. 281–90.
J.K. Wright and I.F. Taylor: Iron Steel Inst. Jpn. Int., 1993, vol. 33 (5), pp. 529–38.
F. Neumann, H. Scheneck, and W. Petterson: Giesserei, 1960, vol. 47, pp. 25–95.
S.K. Khanna, C. Wu, and V. Sahajwalla: High Temp. Mater. Proc., 1998, vol. 17 (3), pp. 193–201.
V. Sahajwalla and R. Khanna: Scand. J. Metall, 2000, vol. 29, pp. 114–20.
F. McCarthy: Ph.D. Thesis, The University of New South Wales, Sydney, Australia, 2005.
J. Morris and D. Buehl: J. Met., 1950, vol. 188, pp 317–25.
V. Sahajwalla and R. Khanna: Yazawa Int. Symp., San Diego, CA, 2003, pp. 825–40.
P.B. Hirch: Electron Microscopy of Thin Crystals, Butterworths, Washington, DC, 1965.
K. Wiik: Ph.D. Thesis, North Western University, 1990.
F. McCarthy, C. Wu, V. Sahajwalla, and J. Hart: Metall. Trans. B, 2003, vol. 34B pp. 573–80.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khanna, R., McCarthy, F., Sun, H. et al. Dissolution of carbon from coal-chars into liquid iron at 1550 °C. Metall Mater Trans B 36, 719–729 (2005). https://doi.org/10.1007/s11663-005-0075-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-005-0075-3