Abstract
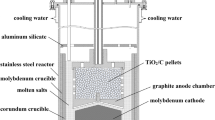
Because of the strong affinity between aluminum and titanium, it has not been possible to produce pure titanium by direct aluminothermic reduction of titanium chlorides. Described in this article is a new process for contactless reduction of titanium dichloride by aluminum in which titanium dichloride and the reductant (aluminum or aluminum alloy) were physically separated, but electrochemically connected through molten NaCl and an external circuit. Titanium dichloride was spontaneously reduced to metal by a cathodic reaction with the simultaneous discharge of chlorine ions into the melt. At the anode, metal aluminum was oxidized to form aluminum chloride dissolved in the molten salt. The electrons were transferred between the electrodes through the external circuit. The concentration of aluminum in titanium produced at 1223 and 1273 K varied from values below the detection limit of the X-ray fluorescence analysis (0.01 mass pct) to 4.5 mass pct. The average contamination was 0.76 mass pct Al. When an aluminum-nickel alloy was used as the reductant, nickel was not detected in the titanium obtained by reduction. This observation suggests that aluminum scrap may be used as a cheap reductant in this contactless electrochemical process.
Similar content being viewed by others
References
W. Kroll: Tr. Electrochem. Soc., 1940, vol. 78, pp. 35–47.
D.R. Sadoway and T.H. Okabe: Massachusetts Institute of Technology, Technology Disclosure, O.S.P. Project No.61243, MIT, Cambridge, MA, 1994.
T.H. Okabe and D.R. Sadoway: J. Mater. Res., 1998, vol. 13, pp. 3372–77.
T. Uda, T.H. Okabe, E. Kasai, and Y. Waseda: J. Jpn. Inst. Met., 1997, vol. 61, pp. 602–09.
T.H. Okabe, T. Uda, E. Kasai, and Y. Waseda: J. Jpn. Inst. Met., 1997, vol. 61, pp. 610–18.
t.H. Okabe and Y. Waseda: J. Met., 1997, vol. 49(6), pp. 28–32.
T. Uda, T.H. Okabe, and Y. Waseda: J. Jpn. Inst. Met., 1998, vol. 62, pp. 76–84.
T. Uda, T.H. Okabe, and Y. Waseda: J. Jpn. Inst. Met., 1998, vol. 62, pp. 796–802.
T. Uda, T.H. Okabe, Y. Waseda, and K.T. Jacob: J. Alloys Compounds, 1999, vol. 284, pp. 282–288.
T.H. Okabe, T. Uda, and Y. Waseda: J. Min. Mater. Processing Inst. Jpn., 1998, vol. 114, pp. 573–79.
F. Zhang, S.L. Chen, Y.A. Chang, and U.R. Kattner: Intermetallics, 1997, vol. 5, pp. 471–82.
T. Yahata, T. Mitsugi, and M. Maeda: CAMP-ISIJ (Proc. Iron Steel Inst. Jpn.), 1990, vol. 3, p. 1646.
G.W. Fletcher: U.S. Patent No. 4,169,722, 1979.
J. Kamlet: U.S. Patent No. 2837426, 1958.
T. Kumagai, S. Konda, T. Sasaki, and T. Ishikawa: Denki Kagaku, 1996, vol. 64, pp. 296–300.
Q. Zhuxian, Z. Minglie, Y. Xaxin, C. Zhenghan, K. Grjothim, and H. Kvande: Aluminium, 1988, vol. 64, pp. 606–09.
M. Maeda, T. Kiwake, K. Shibuya, and T. Ikeda: Mater. Sci. Eng. A, 1997, vols. 239–240, pp. 276–80.
Thermochemical Properties of Inorganic Substances, O. Knacke, O. Kubaschewski, and K. Hesselmann, eds., Springer-Verlag, Berlin, 1991.
O. Kubaschewski and W.A. Dench: J. Inst. Met., 1953–54, vol. 82, pp. 87–91.
K.L. Komarek and M. Silver: Proc. IAEA Symp., Thermodynamics of Nuclear Materials, IAEA, Vienna, 1962, pp. 749–74.
T.H. Okabe, R.O. Suzuki, T. Oishi, and K. Ono: Mater. Trans. JIM, 1991, vol. 32, pp. 485–88.
G.J. Janz: Molten Salts Handbook, Academic Press, New York, NY, 1967.
Binary Alloy Phase Diagrams, 2nd ed., T.B. Massalski, ed., ASM, Materials Park, OH, 1990.
R. Sailer and G. McCathy: Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 44-1294, International Centre for Diffraction Data, Newtown Square, PA, 1993.
H. Linga, K. Motzfeldt, and H.A. Øye: Ber. Bunsenges. Phys. Chem., 1978, vol. 82, pp. 568–76.
S.N. Flengas: Ann. N.Y. Acad. Sci., 1960, vol. 79, pp. 853–72.
V.S. Maksimov and M.V. Smirnov: Electrochem. Mol. Sol. Electrolytes, 1968, vol. 6, pp. 30–36.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uda, T., Okabe, T.H., Waseda, Y. et al. Contactless electrochemical reduction of titanium (II) chloride by aluminum. Metall Mater Trans B 31, 713–721 (2000). https://doi.org/10.1007/s11663-000-0110-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-000-0110-3