Abstract
A new route for the synthesis of the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C high-entropy carbides powders by mechanical alloying is presented. Namely, pure metal powders were wet-milled in toluene using a planetary mill under an argon atmosphere. The synthesized materials crystallize with a rock-salt structure (space group \(Fm{\bar{3}}m\)). A significant reduction in structural defects with annealing of the samples was detected with X-ray diffraction and positron annihilation experiments. Simultaneously, only simple metallic properties without any trace of magnetic or superconducting phase transitions were observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-entropy carbide (HEC) ceramics can be defined as single-phase solid carbide solutions with a random arrangement of four or more different metal cations, each of which has an atomic concentration between 5 and 35 pct, showing the characteristics of crystallographic order but chemical disorder.[1] In recent years, they have attracted considerable interest among physicists and materials scientists for their unique properties. In particular, HECs display high melting points, high hardness, strength, and wear resistance, as well as good anti-corrosion performance.[1,2,3,4,5] This allows them to be used as structural or protective materials in extreme conditions, making them promising for use in the aerospace, mining, and military industries.
To date, HECs with Ti, Zr, Hf, Nb, Ta, Mo, or W metals were successfully fabricated using spark plasma sintering (SPS)[3,6,7] and hot press (HP) sintering.[8,9,10] Unfortunately, these methods are quite expensive, as they require transition metal monocarbides as starting materials, as well as high-temperature (>1900 K) and/or high gas pressure (>10 MPa).[1] In recent years, alternative synthesis methods were investigated. First one in proposed by Chicardi et al.[11,12] According to them, stoichiometric HECs such as (Ti\(_{0.2}\)Zr\(_{0.2}\)Hf\(_{0.2}\)V\(_{0.2}\)Nb\(_{0.2}\))C, (Ti\(_{0.2}\)Zr\(_{0.2}\)Hf\(_{0.2}\)V\(_{0.2}\)Ta\(_{0.2}\))C, (Ti\(_{0.2}\)Zr\(_{0.2}\)Hf\(_{0.2}\)Nb\(_{0.2}\)Ta\(_{0.2}\))C, (Ti\(_{0.2}\)Zr\(_{0.2}\)V\(_{0.2}\)Nb\(_{0.2}\)Ta\(_{0.2}\))C, (Ti\(_{0.2}\)Hf\(_{0.2}\)V\(_{0.2}\)Nb\(_{0.2}\)Ta\(_{0.2}\))C, and (Zr\(_{0.2}\)Hf\(_{0.2}\)V\(_{0.2}\)Nb\(_{0.2}\)Ta\(_{0.2}\))C can be successfully obtained by powder metallurgy at room temperature, specifically by one-step diffusion mechanosynthesis starting from the constituent elements and using pure graphite as a carbon source. A similar method was applied by Moskovskikh et al. to fabricate (Hf\(_{0.2}\)Ta\(_{0.2}\)Ti\(_{0.2}\)Nb\(_{0.2}\)Zr)C and (Hf\(_{0.2}\)Ta\(_{0.2}\)Ti\(_{0.2}\)Nb\(_{0.2}\)Mo\(_{0.2}\))C carbides.[13] Another way to synthesize HECs was proposed by Ning et al.[14] They prepared the stoichiometric (Ta\(_{0.25}\)Nb\(_{0.25}\)Ti\(_{0.25}\)V\(_{0.25}\))C nanopowder by synthesis from molten salts. It should be noted, that although Ning et al. described this method as low-temperature, it actually also requires heating the starting materials at 1537 K in vacuum.
We present a new method for synthesizing HECs by mechanical alloying (MA) powder particles in a high-energy ball mill[15] Similar to the method proposed by Chicardi et al.[11,12] our procedure is based on a room-temperature mechanosynthesis process involving simultaneous alloying of metals and diffusion of carbon into the metal alloy. However, unlike their method, which used pure graphite as the carbon source, we produced new powders of nearly stoichiometric (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C by wet mechanical milling of pure metal powders and toluene, which served as the process control agent and also as the carbon source. In addition, the results of structural and physical characterization of the obtained HECs—both as-milled and annealed in vacuum at high temperature—are presented.
2 Experiments and Data Analysis
The (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C ceramics were synthesized by the mechanical alloying method at room temperature (RT) using a PULVERISETTE 6 planetary ball mill (Fritsch). In particular, commercially available powders of Nb (99.97 pct, Alfa Aesar), Ta (99.6 pct, Alfa Aesar), Ti (99.99 pct, Alfa Aesar), Zr (99.5 pct, Goodfellow), Hf (99.6 pct, Thermo Scientific), Mo (99.95 pct, Alfa Aesar), W (99.9 pct, Alfa Aesar), and pure toluene (Chempur) were utilized as the starting materials. In the first step, the appropriate amounts of metal powders with a total mass of about 10 g were mixed together with 1.2 mL of toluene. The required mass of each metal was calculated from their respective molar fractions: Nb–33.5 pct, Ta–33.5 pct, Ti–11 pct, Hf–11 pct, and Zr–11 pct for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and Nb–33.5 pct, Ta–33.5 pct, Mo–11 pct, Hf–11 pct, and W–11 pct for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C. In the case of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C, the initial ratio of carbon to metal atoms was close to 1.01. For (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C, this ratio was higher and close to 1.12. The 12 pct excess of carbon was used assuming that (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C is more difficult to fabricate than (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. The assumption is based on the calculated entropy-forming ability which is 71 (eV/atom)\(^{-1}\) for HfMoNbTaW carbide and 100 (eV/atom)\(^{-1}\) for HfNbTaTiZr carbide.[2] The material thus prepared and 25 tungsten carbide (WC) grinding balls were then sealed in a cylindrical tungsten carbide vial under an argon atmosphere. The weight ratio of balls to powder was close to 10 to 1, and milling was carried out at room temperature at 300 rpm for 20 hours. After milling, portions of the powders were annealed in a vacuum furnace at 1270 K for 10 days. Finally, it should be noted that after opening the milling vial, there was no scent of toluene and after vacuum annealing, the mass change of both powders was negligible.
X-ray powder diffraction (XRD) patterns for the as-milled and annealed products were collected at RT using a PANalytical X’Pert Pro diffractometer with Cu K\(\alpha \) radiation. The results were analyzed by the Rietveld method using the FULLPROF software package.[16] The mean level of internal strains \(\epsilon \) and the mean grain size L were calculated using the Williamson–Hall method.[17] The chemical composition and homogeneity of the samples were verified by energy-dispersive X-ray spectroscopy (EDXS) using a FESEM FEI Nova NanoSEM 230 scanning electron microscope equipped with an EDAX Genesis XM4 spectrometer.
The X-ray photoelectron spectroscopy (XPS) measurements were carried out in an ultra-high vacuum (UHV) setup equipped with a SPECS Phoibos 150 with standard Mg and Al K\(\alpha \) X-ray sources. The spectrometer was calibrated to yield the typical values of the Au 4f doublet for the clean Au sample. All scans were collected at RT and at a photoelectron take-off angle of 90 deg under the pressure less than \(10^{-9}\) mbar. The measured XPS spectra were analyzed using the CasaXPS program. The background of spectra was subtracted using a software based on Shirley method and the fitting method was the Gaussian-Lorentzian (GL(30 pct)).
Positron lifetime spectra were measured at room temperature with a total of about \(3 \times 10^6\) counts using a conventional fast-fast coincidence system with a resolution of about 300 ps. The \(^{22}\)Na positron source in a Hostaphan polyester envelope was placed between two identical HEC samples, also wrapped in such a film. The lifetime spectra were analyzed using Kansy’s LT-9.0 program.[18]
Magnetic properties of the samples were studied in the temperature range of 1.8 K to 300 K and in magnetic fields up to 1 T using a Quantum Design SQUID magnetometer MPMS XL. Heat capacity was measured from room temperature down to 2 K in zero magnetic field using a Quantum Design’s PPMS platform.
3 Results and Discussion
3.1 Phase Formation and Crystal Structure
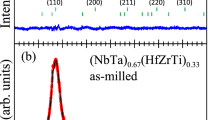
Powder X-ray diffraction patterns of as-milled (a) and annealed (b) (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. Blue dots and black lines represent experimental data and results of the Rietveld refinement, respectively, and red lines show the difference between the two. Green dashes indicate positions of the Bragg reflections (Color figure online)
Powder X-ray diffraction patterns of as-milled (a) and annealed (b) (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C. Blue dots and black lines represent experimental data and the results of the Rietveld refinement, respectively, and red lines show the difference between the two. Green dashes indicate positions of the Bragg reflections (Color figure online)
Figure 1 shows the powder XRD patterns obtained for the as-milled and annealed (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C samples. As can be noticed, both patterns reveal the formation of a face-centered cubic (fcc) phase of the NaCl type (space group \(Fm{\bar{3}}m\)), as in the case of the single carbides NbC, TaC, TiC, HfC, ZrC,[19] and many HECs containing Nb, Ta, Ti, Hf, and Zr (see, e.g., Reference 1). The lattice parameters estimated for the as-milled and annealed samples are a = 4.480(1) Å and a = 4.469(1) Å, respectively. These values are in good agreement with the lattice parameter \(a_{\text{th}}\) = 4.485 Å predicted for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C using Vegard’s law from experimental lattice parameters of the corresponding monocarbides: NbC (4.4544 Å), TaC (4.4530 Å), TiC (4.3247 Å), HfC (4.6309 Å), and ZrC (4.6911 Å).[19]
The mean grain size and the mean level of internal strains were found to be L = 14.5(4) nm and \(\epsilon \) = 0.48(1) pct for the as-milled sample, and L = 22(1) nm and \(\epsilon \) = 0.26(1) pct for the annealed sample. As expected, the thermal treatment of the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C sample leads to an increase in the grain size and a decrease in the lattice parameter and the internal strains level.
In addition, it should be noted that in the case of as-milled (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C, the analysis of the XRD pattern indicates the presence of a small amount of WC (2.3(2) wt pct) with a hexagonal structure and lattice parameters a = 2.906(2) Å and c = 2.840(3) Å (see the lower green ticks in Figure 1(a)). Considering that this HEC does not contain W atoms, it is clear that the formation of that impurity phase is caused exclusively by the abrasion of the vial and grinding balls (made of WC). On the other hand, for the annealed sample, diffraction lines corresponding to WC were absent, while additional diffraction lines, which can be attributed to the cubic, body-centered (bcc) crystal structure of pure W (space group \(Im{\bar{3}}m\), lattice parameter a = 3.1646(1) Å), were observed. This result can be explained taking into consideration the phase diagram of the W–C system presented in works.[20,21] According to these works, at temperatures below 1500 K, the substoichiometric tungsten carbide (WC\(_{\text{1-x}}\)) is not stable and it decomposes into equimolar WC and pure tungsten. Since that, it can be concluded that the as-milled powder contains nanometric grains of metastable substoichiometric WC\(_{\text{1-x}}\) with high concentration of carbon vacancies. During the powder homogenization by the annealing process at 1270 K for 10 days, WC\(_{\text{1-x}}\) decomposes to WC and W. The equimolar WC incorporates into the final HEC due to the solubility of WC in rock salt carbides.[22] At the same time, the XRD pattern measured for the annealed sample indicates the nucleation and further growth of metallic bcc phase of tungsten as the secondary phase in the sample. Finally, it should be mentioned that XRD pattern collected for the annealed sample reveals a set of small peaks within the 2\(\theta \) range from 28 to 32. Those peaks can be ascribed to metal oxides, i.e., HfO\(_2\), ZrO\(_2\), Ta\(_2\)O\(_5\) , or Nb\(_2\)O\(_5\),[22] which were formed in small amount during the vacuum annealing of as-milled sample. The presence of oxygen in the as-milled powder is connected with the fact that before the heat treatment, they had direct contact with atmospheric gases.
The powder XRD patterns obtained for the as-milled and annealed samples of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C are displayed in Figure 2. Analysis of the data showed that the as-milled sample consists mainly of a powder of the desired composition, which exhibits the fcc (NaCl type) crystal structure (similar to the sample with Ti and Zr discussed above). In fact, MoC and WC crystallize in a hexagonal structure. However, as in the case of high-entropy alloys and high-entropy oxides, the prepared multicomponent material is formed in a simple rock salt structure due to the high-entropy stabilization effect.[23] It also contains about 3.1(2) wt pct of WC as an impurity phase. The lattice parameter determined for the fcc phase (a = 4.436(1) Å) turned out to be slightly smaller than the “theoretical” lattice parameter \(a_{\text{th}}\) = 4.457 Å calculated using Vegard’s law. It should be noted here that lattice parameters of WC and MoC with an artificially assumed NaCl-type fcc structure, determined by density functional theory calculations (4.37[24] and 4.39 Å,[25] respectively), were used to estimate \(a_{\text{th}}\).
In turn, the XRD pattern obtained for the annealed sample of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C reveal the presence of the same HEC with identical structure and lattice parameter (space group \(Fm{\bar{3}}m\), a = 4.437(2) Å), as in the as-milled sample, accounting for about 88.6(5) wt pct of the specimen, but with pure W (space group \(Im{\bar{3}}m\), a = 3.1654(2) Å, 11.4(2) wt pct) as an impurity phase. The presence of such a large fraction of pure W in the annealed sample of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C indicates that the synthesized ceramic with Mo and W is less stable at high temperatures than its counterpart with Ti and Zr. In contrast, the effect of the heat treatment on the mean grain size and the internal strains level for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C is comparable to that observed for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. To be specific, L increases from 15.3(6) to 17.0(5) nm, while \(\epsilon \) decreases from 0.97(2) to 0.25(1) pct.
All structural parameters along with the parameters used to assess the quality of the Rietveld refinement,[26] determined for the as-milled and annealed samples of the ceramics (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C, are summarized in Table I. The enlarged XRD patterns in the ranges of 33 to 42 and 67 to 75 deg measured for all studied samples are presented in Electronic Supplementary material (see Figures S1, S2, S3 and S4).
3.2 Vacancy Concentration
The positron annihilation lifetime spectra measured for the as-milled and annealed samples of the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C ceramic are shown in Figure 3. The experimental data can successfully be described by three-component theoretical curves. Two of them, characterized by longer positron mean lifetimes \(\tau _{\text{s1}}\) = 370(5) ps and \(\tau _{\text{s2}}\) = 1700(1) ps, can be attributed to positron annihilation in the Hostaphan film.[27] The third component, characterized by the parameter \(\tau \), describes the annihilation in the studied HEC powders.
As can be noticed in Figure 3, the relative intensity of the contribution coming from the Hostaphan foil is quite high: \(I_{\text{s1}}+I_{\text{s2}}\approx 60 \text{pct}\). This finding can be explained as the result of a combination of two factors. First, in the performed PALS experiments, the Hostaphan film was used to encapsulate not only the \(^{22}\)Na positron source, but also the studied HEC powders. Consequently, one should expect a high probability of positron annihilation in Hostaphan. Second, the XRD data show that the mean grain size in the prepared samples ranges from 14 to 22 nm, which is smaller than the average diffusion length of a thermalized positron.[28] For this reason, the high probability of positron annihilation at particles’ surface and interfaces must be also taken into account. Indeed, according to previous PALS studies of vanadium carbide powders with nanometer grain size, the mean lifetime of positrons that annihilate on the surface of nanocrystals is between 314 and 349 ps.[29] The value calculated from the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C powders spectra, namely \(\tau _{\text{s1}}\) = 370(5) ps, is close to that range. Thus, it can be safely assumed that the \(\tau _{\text{s1}}\)-component of the intensity \(I_{\text{s1}}\) > 50 pct corresponds to positron annihilation in the Hostaphan film and at particles’ surface and interfaces of the HEC in discussion.
PALS spectra measured for as-milled and annealed samples of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C (blue open circles) along with fits described in the text (solid curves) and their residuals (closed circles). Green and purple curves represent contributions of a positron source holder (\(\tau _{\text{s1}}\) and \(\tau _{\text{s2}}\)), and red curves represent sample contributions with \(\tau \) (Color figure online)
PALS spectra measured for as-milled and annealed samples of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C (blue open circles) along with fits described in the text (solid curves) and their residuals (closed circles). Green and purple curves represent contributions of a positron source holder (\(\tau _{\text{s1}}\) and \(\tau _{\text{s2}}\)), and red curves represent sample contributions with \(\tau \) (Color figure online)
The most important component of the fit shown in Figure 3 is the third one, described by the the shortest lifetime \(\tau \), which characterizes the annihilation of positrons in the bulk of the studied samples of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. For the as-milled powder, \(\tau \) = 174(11) ps, while for the annealed sample, it decreases significantly to 115(18) ps. These values can be compared with positron annihilation lifetime data for transition metal carbides which are presented in Reference 30. In particular, ab initio calculations of the electron structure and positron states carried out for idealized lattices of the carbides TiC, HfC, ZrC, NbC, and TaC yielded \(\tau _{\text{bulk}}\) values between 98 and 114 ps. For lattices with metal or carbon vacancies (as in the real samples studied), the positron lifetimes are much longer due to strong positron trapping in these defects. In addition, it should be noted that the theoretical calculations show that the decrease in positron affinity caused by metal vacancies is stronger than by vacancies in carbon. As a result, the mean values of the theoretical positron lifetimes corresponding to the vacancies at metal sites \(\tau _{\text{v-M}}\) are from 161 to 196 ps, while for the vacancies at carbon sites \(\tau _{\text{v-C}}\) , they are lower and range from 124 to 148 ps.
Taking the above into account, it can be concluded that \(\tau \) = 174(11) ps obtained for the as-milled powder of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C indicates that this sample contains a high concentration of vacancies at metal and carbon sites. At the same time, annealing the ceramic at 1270 K for 10 days resulted in a significant reduction in the concentration of both types of vacancies giving \(\tau \) = 115(18) ps, which is much closer to \(\tau _{\text{bulk}}\) than to \(\tau _{\text{v-M}}\) and \(\tau _{\text{v-C}}\). It should be noted here, however, that \(\tau \) = 115(18) ps is still slightly higher than the average lifetime \(\tau _{\text{av}}\) = 102 ps calculated using the mixing rule and theoretical values of \(\tau _{\text{bulk}}\) for idealized lattices of the TiC, HfC, ZrC, NbC, and TaC carbides. Therefore, a small concentration of the carbon vacancies and/or other types of defects (i.e., edge and screw dislocations) probably remain in the annealed powder of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. Our findings are consistent with the results of the XRD data analysis, which show that after heat treatment, the mean level of internal strains decreases from 0.48 to 0.26 pct.
The PALS spectra measured for the as-milled and annealed samples of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C are shown in Figure 4. Similar to the results obtained for the HEC with Ti and Zr, the two spectra recorded for the ceramic with Mo and W can be described by three components: two related to the positron annihilation in the Hostafan film and at the HEC particles’ surface and interfaces (\(\tau _{\text{s1}}\) and \(\tau _{\text{s2}}\)) and one associated with positron annihilation in the bulk of the alloy under study (\(\tau \)). The latter value for the as-milled powder of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C is 168(12) ps, while for the annealed powder, it is 107(14) ps. These values are comparable with \(\tau \) obtained for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C, thereby indicating that the as-milled sample of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C contains a significantly higher concentration of carbon and metal vacancies than the annealed sample.
Note that both lifetimes determined for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C are slightly shorter than corresponding lifetimes for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. This difference is probably due to the fact that positron annihilation lifetimes for Ti (150 ps) and Zr (164 ps) are much longer than for Mo (106 ps) and W (105 ps).[31] Consequently, a lower \(\tau \) value should be expected for HECs with Mo and W atoms than for alloys with Ti and Zr.
3.3 Homogeneity and Chemical Composition
Mapping of the elements in the as-milled sample of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C (Figure 5) shows that the alloy is a microscopically homogeneous mixture of the constituent elements, with no zones significantly enriched in any of the components. And its atomic (chemical) composition was found to be close to: Nb–16.9(1) pct, Ta–16.3(1) pct, Ti–5.0(1) pct, Hf–5.5(1) pct, Zr–7.3(1) pct, and C+O–49(10) pct. At the same time, the relative atomic ratio of the metal components only (normalized to 100 pct), which can be determined with much better precision, is: Nb–33 pct, Ta–32 pct, Ti–10 pct, Hf–11 pct, Zr–14 pct, which is close to the nominal one, i.e., approximately Nb–33.5 pct, Ta–33.5 pct, Ti–11 pct, Hf–11 pct, and Zr–11 pct.
It should be noted here that due to the relatively low accuracy of quantitative EDXS measurements of light elements (such as oxygen and carbon) the concentrations of these elements have been determined with large uncertainties. Moreover, the emission spectra from oxygen and carbon overlap, so only the summed value for C+O is given. Nevertheless, it seems reasonable to assume that the oxygen content of the prepared sample is less than 5 at. pct, and that the oxygen atoms are mainly on the surface of the powder particles and in the grain boundaries. First, given that the starting materials and toluene did not contain oxygen, and that the milling was carried out under a protective argon atmosphere, noticeable oxidation of the sample during synthesis is unlikely. Second, according to Reference 13, the oxygen concentration determined by an alternative method of chemical analysis (gas analyzer) for the carbides (HfTaTiNbZr)C\(_5\) and (HfTaTiNbMo)C\(_5\) fabricated through reactive high-energy ball milling followed by spark plasma sintering was lower than 5 at. pct. At the same time, EDXS analysis showed that the oxygen content of their samples was higher than 10 at. pct. Third, the XRD patterns obtained for our as-milled samples (Figures 1, 2) do not show reflections attributable to metal oxides. Our hypothesis has also been further verified in the XPS measurements, which is a surface-sensitive technique (see Section III–D).
In the case of the as-milled sample of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C, the SEM/EDX analysis demonstrates a homogeneous distribution of the elemental components (Figure 6) and yields the atomic composition: Nb–18.4(1) pct, Ta–18.1(1) pct, Mo–5.4(1) pct, Hf–5.9(1) pct, W–8.2(1) pct, and C+O–44(10) pct. In turn, the relative atomic ratio of the metal components only (normalized to 100 pct) was found to be: Nb–33 pct, Ta–32 pct, Mo–10 pct, Hf–10 pct, and W–15 pct, which again is close to the nominal composition of the alloy.
Mapping of the elements in the annealed samples (presented in Electronic Supplementary Material in Figures S5 and S6) shows that the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C is microscopically homogeneous, while in the case of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C, some regions rich in tungsten are observed. This result is in agreement with XRD data.
3.4 Surface Characterization
The surface chemical composition and the chemical state of the constituent elements in the surface layer of the prepared powders were studied by XPS. Before inserting the as-milled HEC samples into UHV chamber, both powders were pressed into copper pellets. Additionally, both HEC samples had direct contact with atmospheric gases. Therefore, in order to reduce the surface contamination, the XPS measurements were performed after annealing the powders at 940 K for 105 minutes in UHV. The annealing temperature is limited by the melting temperature of the copper pellets.
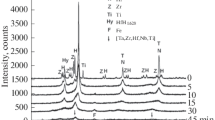
Figure 7 shows six selected high-resolution XPS spectra measured for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C powder. As one can notice, hafnium and tantalum signals occur in the same binding energy (BE) region, while niobium, zirconium, carbon, and oxygen signals are detected separately. Surprisingly, the wide-range XPS spectrum does not reveal the presence of the signal peak of titanium. The first envelope which is presented in Figure 7(a) contains overlapping Ta 4d doublets and Hf 4d\(_{3/2}\) peaks. Detailed deconvolution of this spectrum permitted distinction of four tantalum doublet components and two hafnium peaks. In the case of Ta 4d\(_{5/2}\) peaks, they can be attributed to metallic Ta\(^0\) at the BE of 226.2 eV, TaC at the BE of 227.8 eV and two Ta oxides species placed at 229.8 eV (TaCO) and 231.4 eV (TaO\(_{\text{x}}\)).[32,33] Correspondingly, the Ta 4d\(_{3/2}\) envelope is composed of four tantalum components, located at 237.7, 239.5, 242.2, and 244.3 eV. In the case of hafnium signals, which occur at the lower binding energy site in Figure 7(a), one can identify HfC component at the BE of 222.5 eV and HfO\(_{\text{x}}\) at 224.6 eV.[34] XPS spectrum taken at low BE region which is composed of Hf 4f, Ta 4f, and O 2s signals is shown in Figure 7(b). The detailed analysis of this spectrum reveals the presence of similar components to those observed for Ta 4d and Hf 4d signals. The XPS Zr 3p spectrum presented in Figure 7(c) can be described using two doublets, from which the first represents the Zr\(^0\) state (330.0 eV for 3p\(_{3/2}\) and 347.4 eV for 3p\(_{1/2}\)), while the second with maxima located at 333.3 and 351.0 eV corresponds to ZrC.[34] In the case of Nb 3d core level line (Figure 7(d)), signals from carbide and oxygen species are detected. In particular, the dominant NbC doublet is observed at the BE of 203.8 eV (3d\(_{5/2}\)) and 206.5 eV (3d\(_{3/2}\)), while NbO\(_{\text{x}}\) is represented by peaks located at 204.8 eV (3d\(_{5/2}\)) and 207.7 eV (3d\(_{3/2}\)).[33,35] The C 1s XPS spectrum (Figure 7(e)) is composed of five peaks. On the lower side of the binding energy range, at the position of 282.7 eV, one can identify a peak which corresponds to metal carbides.[33,34] The peak with the highest intensity located at the BE of 284.3 eV represents free-carbon contamination on the surface (graphite-like) and on the higher BE site there are three components corresponding to CO, CO\(_2\) , and hydrocarbons groups.[32,34] Finally, the O 1s XPS spectrum (Figure 7(f)) contains two peaks, from which the dominant one, at the BE of 530.7 eV, is related to the metal oxides, while the second one, at the BE of 532.2 eV, is ascribed to strongly adsorbed oxygen.[32,34]
The detailed analysis of the XPS spectra displayed in Figure 7 reveals the presence of metal carbides, oxides, and oxycarbides as well as CO, CO\(_2\), hydrocarbons groups, and even a small amount of metallic Zr and Ta. Connecting these findings with XRD and SEM data, one can state that the core of the prepared particles is composed of NaCl-type fcc-structured HEC phase, while their surface is covered by a thin layer which is a mixture of carbides, oxides, oxycarbides, and adsorbed gases. This finding is probably connected with a high level of C and O surface contamination which comes from the method of synthesis (MA with toluene) and from direct contact of prepared HEC particles with air before XPS measurements. Additionally, these results clearly show that the annealing in UHV at 940 K is insufficient to effectively remove adsorbed CO, CO2, and hydrocarbons from the powder’s surface.
Six selected high-resolution XPS spectra measured for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C powder are presented in Figure 8. The detailed analysis of the XPS signal envelope in the 218 to 234 eV BE range allows to distinguish seven components (Figure 8(a)). Similarly, like for the previous sample, the first two peaks, at the BE of 222.2 and 224.2 eV, are attributed to the Hf 4d\(_{3/2}\) signals from HfC and HfO\(_{\text{x}}\), respectively.[34] The third and fourth peaks, at the BE of 225.7 and 227.0 eV, may be connected with slightly shifted to the lower BE range signals from Ta\(^0\) and TaC (Ta 4d\(_{5/2}\)).[33] The next three components are ascribed to signals from tantalum and molybdenum which overlap each other. It can be suggested that the component at the position of 228.1 eV is related to the Mo\(^0\) species[36] and simultaneously to TaCO\(_{\text{x}}\). Similarly, another peak with the BE of 229.5 eV may be assigned to MoC[36,37] and some amounts of Ta oxides. The last component, placed at the highest BE site, at the position of 231.4 eV, is related to the Mo oxide species, like MoCO\(_{\text{x}}\) and MoO\(_{\text{x}}\).[37] In the case of Ta 4d\(_{3/2}\) doublet component, the analysis is also complicated due to the presence of W 4d signal (Figure 8(b)). The peaks located at the BE of: 237.3, 238.8, and 240.9 eV correspond to Ta\(^0\), TaC, and TaCO\(_{\text{x}}\) species, respectively.[32,34] The component, with the maximum at the BE position of 243.3 eV, is related to the presence of metallic W in the measured region[38] and can be also assign to Ta oxides. Another component located at the BE of 244.3 eV may represent both tantalum and tungsten species, like WC[37,38] and TaO\(_{\text{x}}\),[34] while the last peak, at the BE of 246.4 eV, represents only tungsten oxides (WO\(_{\text{x}}\)).[37,38] Additionally performed measurements of tantalum Ta 4f and hafnium XPS Hf 4f signals, presented in Figure 8(c), confirm previous analyses. Core level line exhibits two broadened multicomponent peaks representing various species. In the case of XPS Nb 3d core level line (Figure 8(d)), only one doublet corresponding to the NbC is observed at the position of 203.8 eV (3d\(_{5/2}\)) and 206.6 eV (3d\(_{3/2}\)).[33] The shape of the carbon core level line (Figure 8(e)) differs from the one measured for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C powder. Instead, three components are distinguished, from which the most dominant one is again C-C peak (graphite-like) at the BE of 284.3 eV.[39] However, the relative contribution of the carbide component located at 283.0 eV to all three components observed in this region is close to 33 pct and this value is much higher than 18 pct determined for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C (Figure 7(e)). The last peak in C 1s spectrum, placed at the BE of 286.0 eV, corresponds to C–O bonding.[39] The O 1s XPS spectrum contains three peaks (Figure 8(f)), from which the first, at the BE of 530.7 eV, corresponds to the metal oxides, while the others, at the BE of 532.2 and 533.6 eV, are ascribed to strongly adsorbed oxygen.[39]
Generally, the XPS results obtained for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C powder are comparable with those for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C. The (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C particles are covered by a thin layer mainly composed of metal carbides, oxides, and oxycarbides. However, the high relative contribution of the carbide component in XPS C 1s spectrum (Figure 8(e)) suggests that, in this case, the surface concentration of metal carbides is much higher in comparison with (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C particles. This effect may be connected with a different metallic element composition of both powders. In particular, Zr and Ti metals have a much higher affinity for oxygen than W and Mo.[40] Therefore, it is reasonable to expect that the surface of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C particles would contain a higher concentration of oxides and oxycarbides than (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C. At this point, it must be strongly emphasized that the presence of metal oxides and oxycarbides in both studied HEC powders is limited to the particles surface. This statement is based on recent study by Lun et al. of oxidation behavior of various non-stoichiometric (ZrHfTi)C\(_{\text{x}}\) carbide solid solution powders in air.[41] According to their work, (ZrHfTi)C\(_{\text{x}}\) carbides gradually transform during oxidation process to m-(ZrHfTi)O\(_2\) and TiO\(_2\) with the increase in an oxidation temperature. None of these phases were detected in XRD patterns presented in Figures 1 and 2.
3.5 Magnetic Properties
Molar magnetic susceptibilities \(\chi _{\text{mol}}\) of the as-milled and annealed samples of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C measured as a function of temperature are plotted in Figures 9 and 10, respectively. As can be seen, both alloys before the annealing are Pauli paramagnets with an almost temperature interdependent \(\chi _{\text{mol}}(T)\) and some Curie-like contribution from unavoidable paramagnetic impurities, evident at the lowest temperature studied. After annealing, the susceptibility of both the alloy with Ti and Zr and the one with Mo and W decreases significantly and even becomes negative at room temperature. This means that the annealed alloy is actually diamagnetic, and the apparent change in the sign of the susceptibility to positive is due to the presence of the paramagnetic impurities. Incidentally, concentration of these impurities is much lower in the annealed sample than in the as-milled one.
Temperature dependence of molar magnetic susceptibility \(\chi _{\text{mol}}\) of as-milled and annealed (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C powders measured in an applied magnetic field \(\mu _0H\). Black solid lines represent fits of Eq. [1] to experimental data. Inset: isothermal field dependence of mass magnetization \(\sigma \)
Temperature dependence of molar magnetic susceptibility \(\chi _{\text{mol}}\) of as-milled and annealed (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C powders measured in an applied magnetic field \(\mu _0H\). Black solid lines represent fits of Eq. [1] to experimental data. Inset: isothermal field dependence of mass magnetization \(\sigma \)
These results can be compared with the magnetic properties of transition metal carbides and binary carbides M\(_{1-x}\)M\(_x^{\prime }\)C (where M, M\(^{\prime }\) = Ti, Hf, Zr, Nb, and Ta), which were comprehensively discussed by Dubrovskaya and Nazarova in Reference 42. According to their work, magnetic susceptibility measured at room temperature is positive for NbC, TaC, and TiC, and negative for HfC and ZrC. For this reason, the resultant susceptibility of the M\(_{1-x}\)M\(_x^{\prime }\)C alloys is strongly dependent on their composition. In addition, the \(\chi (T)\) data obtained for the NbC and TaC carbides in the range from 100 K to 1200 K reveal that the magnetic susceptibility increases slightly with temperature for the former and decreases for the latter.
This effect was explained by temperature smearing of the Fermi surface in the studied carbides.[42] In particular, when the Fermi level \(E_{\text{F}}\) is near the minimum of the DOS function N(E), the coefficient \({d\chi }/{dT}\) is positive and the Pauli paramagnetic susceptibility increases with increasing temperature. On the other hand, when \(E_{\text{F}}\) lies near the maximum of N(E), \({d\chi }/{dT}\) is negative. It is worth noting that this interpretation does not contradict the Pauli paramagnetism model. This is because it assumes an idealized shape of the DOS curve, while in reality, the electron band structure is very complex.
Given the above, the experimental \(\chi _{\text{mol}}(T)\) data can be approximated by an equation of the form:
where \(\chi _0\) is the temperature-independent contribution to the magnetic susceptibility of the majority phase, \(\alpha \equiv {d\chi }/{dT}\) is the temperature coefficient describing the aforementioned contribution from the smearing of the Fermi level of the studied alloy, and the third term (with the Avogadro number \(N_{\text{A}}\), the Boltzmann constant \(k_{\text{b}}\), the magnetic effective moment \(\mu _{\text{eff}}\), and the Curie–Weiss temperature \(\theta _{\text{p}}\)) describes the Curie-like properties of the magnetic impurities. Least-squares fits of Eq. [1] to the experimental data yielded the parameters given in Table II.
As can be seen, the effective magnetic moments obtained from the fits are very small in each case and do not vary much from sample to sample. This is consistent with our hypothesis that they come from small amounts of unavoidable paramagnetic impurities that are virtually impossible to detect by other methods. In other words, the actual effective moments of the impurities (e.g., rare earths elements) are two to three orders of magnitude larger, but their small amounts give small values of \(\mu _{\text{eff}}\) per formula unit).
At the same time, the \(\chi _0\) and \(\alpha \) parameters decrease significantly with thermal treatment of the investigated samples. This effect may be related to the magnetic properties of stoichiometric and non-stoichiometric transition metal monocarbides, reported by Bittner and Goretzki.[43,44] They found that magnetic susceptibility measured for NbC, TaC, TiC, ZrC, and HfC is of the order of 0.1\(\times \)10\(^{-9}\) m\(^3\)mol\(^{-1}\) (as in the HEC alloys discussed here; see Table II). However, these values strongly depend on the ordering of carbon atoms and vacancies in the non-metallic fcc sublattice, i.e., the susceptibility of TiC, HfC, and ZrC monocarbides increases abruptly as the level of disorder and concentration of the carbon vacancies increases.[43,45] The decrease in the \(\chi _0\) parameter observed for the (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C alloys after the heat treatment (see Figures 9 and 10, and Table 2) can therefore be explained by assuming that the annealed samples contain fewer defects (vacancies, dislocations, and grain boundaries) and a lower level of internal deformation than the as-milled samples. This assumption seems to be in full agreement with the XRD and PALS results described in Sections III–A and III–B.
The field dependence of the magnetization \(\sigma \) measured at 1.8 K for the as-milled and annealed samples of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C is shown in the insets of Figures 9 and 10, respectively. The overall shape of the \(\sigma (H)\) curves and the magnetization values reflect the paramagnetic nature of the alloys studied. Interestingly, no trace of any negative contribution which could indicate the presence of superconductivity was found in magnetic fields approaching zero.
The latter result is quite unexpected, since TaC, NbC, and TiC are superconductors with critical temperatures \(T_{\text{c}}\) of 10.4 K, 10.5 K, and 0.5 K, respectively.[42,46,47] The same is true for WC and MoC with NaCl-type fcc structures, which exhibit relatively large \(T_{\text{c}}\) exceeding 10 K.[48] The superconductivity has not been found only in the case of ZrC (down to 1.28 K) and HfC (down to 0.015 K).[49] As for high-entropy alloys related to the HECs described in this paper, the superconductivity has been observed in (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\) (\(T_{\text{c}}\) = 7.3 K[50]) and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\) (\(T_{\text{c}}\) = 4.3 K[51]), which crystallize, however, in a bcc structure. On the other hand, there are no data in literature on the occurrence of the superconductivity in HECs containing Nb, Ta, Ti, Hf, Zr, Mo, or W.[1,52] One can find only a few, very recent papers that have reported on the superconductivity in the medium-entropy alloy with added carbon, namely MoReRuC\(_x\),[53] and high-entropy alloy (MoReRu)\(_{(1-2x)/3}\)(PdPt)\(_x\)C\(_y\).[54]
3.6 Thermodynamic Properties
The temperature dependence of the specific heat \(C_{\text{p}}\) measured for the annealed samples of (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C is shown in Figures 11 and 12, respectively. As can be seen, the \(C_{\text{p}}(T)\) curves obtained for both materials are very similar. In particular, they do not show any anomaly that could indicate magnetic ordering or a transition to a superconducting state, which agrees perfectly with the magnetic susceptibility data.
Specific heat \(C_{\text{p}}(T)\) of annealed (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C as a function of temperature; solid curve represents a fit of Eq. [1] to the experimental data. Lower inset: low-temperature \(C_{\text{p}}/T\) vs \(T^2\); solid line is a fit of Eq. [2] to the experimental data. Upper inset: temperature variation of phonon contribution \((C_{\text{p}}-\gamma T)\) divided by \(\beta T^3\)
Specific heat \(C_{\text{p}}(T)\) of annealed (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C as a function of temperature; solid curve represents a fit of Eq. [1] to the experimental data. Lower inset: low-temperature \(C_{\text{p}}/T\) vs \(T^2\); solid line is a fit of Eq. [2] to the experimental data. Upper inset: temperature variation of phonon contribution \((C_{\text{p}}-\gamma T)\) divided by \(\beta T^3\)
Below about 6 K, the experimental data can be described by the sum:
where the first term is the well-known electron specific heat in the Sommerfeld model of free electrons, and the second term is the low-temperature approximation of the phonon specific heat in the Debye model. Least-squares fits of Eq. [2] to the experimental data (see the lower insets of Figures 11 and 12) gave the parameters \(\gamma \) = 2.1(1) mJ K\(^{-2}\)mol\(^{-1}\) and \(\beta \) = 0.026(1) mJ K\(^{-4}\)mol\(^{-1}\) for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C, and \(\gamma \) = 2.2(1) mJ K\(^{-2}\)mol\(^{-1}\) and \(\beta \) = 0.029(1) mJ K\(^{-4}\)mol\(^{-1}\) for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C.
The estimated \(\gamma \) values are significantly lower than those obtained for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\) (8.3 mJ K\(^{-2}\)mol\(^{-1}\)[50]) and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\) (4.8 mJ K\(^{-2}\)mol\(^{-1}\),[51]) indicating lower density of states at the Fermi level (\(N(E_{\text{F}})\)). Interestingly, similar behavior has been observed for transition metal elements and their monocarbides. As suggested, two d-like subbands with high density of states in the band structure of transition metals become energetically separated and shifted far apart in their carbides, and a conduction band with low density of d states is formed between the two subbands.
At the same time, the \(\gamma \) value found for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C agrees perfectly with the theoretical parameter \(\gamma _{\text{th}}\) = 2.2 mJ K\(^{-2}\) mol\(^{-1}\), calculated according to the rule of mixtures and using the experimentally determined \(\gamma \) values of the monocarbides, namely: 2.83 mJ K\(^{-2}\) mol\(^{-1}\) (NbC), 3.2 mJ K\(^{-2}\) mol\(^{-1}\) (TaC), 0.51 mJ K\(^{-2}\) mol\(^{-1}\) (TiC), 0.75 mJ K\(^{-2}\) mol\(^{-1}\) (ZrC), and 0.75 mJ K\(^{-2}\) mol\(^{-1}\) (HfC).[44] In the case of (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C, the \(\gamma _{\text{th}}\) parameter cannot be estimated becuase the \(\gamma \) value of WC with the NaCl-type (fcc) structure is unknown.
The experimental \(\gamma \) can be used to estimate \(N(E_{\text{F}})\) and the Pauli susceptibility of the conduction electrons, \(\chi _{\text{P}}\), from the equations:
and
For both HECs studied, \(N(E_{\text{F}})\) = 0.9(1) states eV\(^{-1}\) per f.u., while \(\chi _{\text{P}}\) is of about 0.3(1)\(\times \)10\(^{-9}\) m\(^3\)mol\(^{-1}\).
Assuming additivity of magnetic quantities, the temperature-independent susceptibility \(\chi _0\) determined from the \(\chi (T)\) data (see Section III–E) can be expressed by the sum:
where \(\chi _{\text{core}}\) is the isotropic diamagnetic susceptibility of localized core electrons, \(\chi _{\text{VV}}\) is the averaged anisotropic paramagnetic Van Vleck susceptibility, and \(\chi _{\text{L}}\) is the isotropic Landau diamagnetic susceptibility of the conduction electrons. Since the \(\chi _{\text{VV}}\) contribution is positive and the absolute value of the diamagnetic susceptibility \(\chi _{\text{core}}\) is lower than 0.1\(\times \) 10\(^{-9}\) m\(^3\) mol\(^{-1}\),[55] the negative values of \(\chi _0\) observed for both annealed HEC samples (Table 2) can only be explained by the Landau diamagnetism. It can be expresses as:
where \(m_0\) is the mass of the free electron, and \(m^*\) is the effective mass of a charge carrier on the Fermi surface. Having the values of \(\chi _{\text{P}}\) and \(\chi _0\), and assuming that \(\chi _{\text{core}}+\chi _{\text{VV}}+\chi _{\text{P}} > 0\), one can easily deduce that the ratio \(m^*/m_0\) must be lower than 1 for the annealed samples.
From the determined \(\beta \) values, the characteristic Debye temperature \(\Theta _{\text{D}}^{\text{LT}}\) of the studied alloys can be estimated (the superscript LT indicates that the parameter was estimated from low-temperature data) using the formula:
where R is the universal gas constant, and r is the number of atoms per formula unit (r = 2 for both HECs studied). This yielded \(\Theta _{\text{D}}^{\text{LT}}\) = 531(7) K for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and \(\Theta _{\text{D}}^{\text{LT}}\) = 512(6) K for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C.
In addition, the obtained \(\gamma \) and \(\beta \) values can be used to describe \(C_{\text{p}}(T)\) data over the entire temperature range studied. First, however, it should be noted that for both HECs, a characteristic anomaly appears in the form of a low-temperature peak in the non-electron heat capacity \((C_{\text{p}}-\gamma T)/\beta T^3\) plotted as a function of T (see upper insets in Figures 11 and 12). This anomaly is a proof that the Debye model with one characteristic temperature as a fitting parameter cannot even roughly describe the experimental curve. Usually, such low-temperature specific heat in excess of the Debye model is attributed to scattering of acoustic phonons or excitation of optical phonons[56] and can be described by adding the Einstein modes to the considered model (note that the position of the maximum in the experimental curve corresponds to 1/5 of the characteristic Einstein temperature \(\Theta _{\text{E}}\)). Accordingly, the \(C_{\text{p}}(T)\) data collected for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C were analyzed taking into account the electron contribution, Einstein function, and Debye model (further corrected for anharmonicity[57]), represented by the first, second, and third terms in the formula, respectively:
\(\Theta _{\text{D}}^{\text{HT}}\) is the Debye temperature (the superscript HT indicates that the parameter was estimated from high-temperature data), \(n_{\text{D}}\) and \(n_{\text{E}}\) are the numbers of Debye- and Einstein-type modes, respectively (\(r = n_{\text{E}} + n_{\text{D}} = 2\)), and \(\alpha \) is the parameter describing the anharmonicity of the system. Least-squares fits of Eq. [8] to the experimental data yielded the following values of the parameters: \(\alpha \) = 1.4(2)\(\times \)10\(^{-4}\) K\(^{-1}\), \(\Theta _{\text{D}}^{\text{HT}}\) = 947(8) K, \(\Theta _{\text{E}}\) = 210(2) K, \(n_{\text{D}}\) = 1.28(1) and \(n_{\text{E}}\) = 0.72(1) for (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C (see the solid curve in the main panel of Figure 11) and \(\alpha \) = 1.04(3) \(\times \)10\(^{-3}\) K\(^{-1}\), \(\Theta _{\text{D}}^{\text{HT}}\) = 1024(11) K, \(\Theta _{\text{E}}\) = 204(1) K, \(n_{\text{D}}\) = 1.21(1) and \(n_{\text{E}}\) = 0.79(1) for (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C (see the solid curve in the main panel of Figure 12).
As can be seen, for the two alloys studied, the estimated respective values of \(\Theta _{\text{D}}^{\text{HT}}\) and \(\Theta _{\text{D}}^{\text{LT}}\) differ significantly from each other. Some divergence of these values is indeed expected, mainly due to the weakness of the Debye model (see, e.g., Reference 58). Moreover, we have included more physical phenomena in the second approach (Eq. [8]) than in the first (Eq. [2]). Nevertheless, the observed differences in the Debye temperatures obtained from the low- and high-temperature fittings can still be counted as surprisingly large. According to Reference 59, the Debye temperatures determined for TaC, NbC, ZrC, and HfC are also dependent on the temperature range considered. For example, \(\Theta _{\text{D}}\) for TaC is close to 400 K at low temperatures and rises to nearly 800 K at room temperature. Therefore, it can be assumed that a similar temperature dependence of the \(\Theta _{\text{D}}\) parameter can be observed in the case of the studied HECs.
Finally, it should be noted that comparing the estimated values \(\Theta _{\text{D}}\) with results of theoretical calculations is difficult, because the calculated values of \(\Theta _{\text{D}}\), which can be found in the literature,[46,60,61] differ significantly from each other. For example, the Debye temperature for NbC, depending on the Reference, is estimated to be 328 K ,[46] 779–860 K,[60] or 680 K.[61]
4 Conclusions
A new route for synthesis of high-entropy carbides via mechanical alloying in a high-energy ball mill was presented. In result, two new (NbTa)\(_{0.67}\)(TiHfZr)\(_{0.33}\)C and (NbTa)\(_{0.67}\)(MoHfW)\(_{0.33}\)C HEC powders were fabricated through wet mechanical milling of pure metal powders and toluene which served as the process control agent and also as the carbon source. The prepared samples had the NaCl-type crystal structure with small amount of secondary phases. The values of lattice parameter estimated for fcc phase by the Rietveld method are comparable to those determined for Nb, Ta, Ti, Hf, and Zr monocarbides as well as many HEC containing these metals. The elemental mapping showed that the prepared HEC powders are microscopically homogeneous mixture of the constituent elements with no zones significantly enriched in any of the components. However, their surface is covered by a thin layer mainly composed of metal carbides, oxides, and oxycarbides. The magnetic measurements reveal that both studied materials are Pauli paramagnets with an almost temperature interdependent \(\chi _{\text{mol}}(T)\) and some Curie-like contribution from unavoidable paramagnetic impurities. Finally, the low-temperature magnetic and specific heat data point to the absence of superconductivity down to 1.8 K.
Data Availability
The data presented in this study are openly available in OSF repository at DOI 10.17605/OSF.IO/G4N6B.
References
Y. Wang: Adv. Appl. Ceram., 2022, vol. 121(2), pp. 57–78. https://doi.org/10.1080/17436753.2021.2014277.
P. Sarker, T. Harrington, C. Toher, C. Oses, M. Samiee, J.-P. Maria, D. Brenner, K. Vecchio, and S. Curtarolo: Nat. Commun., 2018, vol. 9, p. 4980. https://doi.org/10.1038/s41467-018-07160-7.
E. Castle, T. Csanádi, S. Grasso, J. Dusza, and M. Reece: Sci. Rep., 2018, vol. 8, p. 8609. https://doi.org/10.1038/s41598-018-26827-1.
C. Peng, X. Gao, M. Wang, L. Wu, H. Tang, X. Li, Q. Hang, Y. Ren, F. Zhang, Y. Wang, B. Zhang, B. Gao, Q. Zou, Y. Zhao, Q. Yang, D. Tian, H. Xiao, H. Gou, W. Yang, X. Bai, W.L. Mao, and H.-K. Mao: Appl. Phys. Lett., 2019, vol. 114(1), p. 011905. https://doi.org/10.1063/1.5054954.
Y. Wang, T. Csanádi, H. Zhang, J. Dusza, M.J. Reece, and R.-Z. Zhang: Adv. Theory Simul., 2020, vol. 3(9), p. 2000111. https://doi.org/10.1002/adts.202000111.
T.J. Harrington, J. Gild, P. Sarker, C. Toher, C.M. Rost, O.F. Dippo, C. McElfresh, K. Kaufmann, E. Marin, L. Borowski, P.E. Hopkins, J. Luo, S. Curtarolo, D.W. Brenner, and K.S. Vecchio: Acta Mater., 2019, vol. 166, pp. 271–80. https://doi.org/10.1016/j.actamat.2018.12.054.
J. Dusza, T. Csanádi, D. Medved, R. Sedlák, M. Vojtko, M. Ivor, H. Ünsal, P. Tatarko, M. Tatarková, and P. Šajgalík: J. Eur. Ceram. Soc., 2021, vol. 41(11), pp. 5417–26. https://doi.org/10.1016/j.jeurceramsoc.2021.05.002.
B. Ye, T. Wen, K. Huang, C. Wang, and Y. Chu: J. Am. Ceram. Soc., 2018, vol. 102, p. 16295. https://doi.org/10.1111/jace.16295.
L. Feng, W.G. Fahrenholtz, and G.E. Hilmas: J. Am. Ceram. Soc., 2019, vol. 102(12), pp. 7217–24. https://doi.org/10.1111/jace.16672.
K. Wang, L. Chen, C. Xu, W. Zhang, Z. Liu, Y. Wang, J. Ouyang, X. Zhang, Y. Fu, and Y. Zhou: J. Mater. Sci. Technol., 2020, vol. 39, pp. 99–105. https://doi.org/10.1016/j.jmst.2019.07.056.
E. Chicardi, C. García-Garrido, and F.J. Gotor: Ceram. Int., 2019, vol. 45(17), pp. 21858–63. https://doi.org/10.1016/j.ceramint.2019.07.195.
E. Chicardi, C. García-Garrido, J. Hernández-Saz, and F.J. Gotor: Ceram. Int., 2020, vol. 46(13), pp. 21421–30. https://doi.org/10.1016/j.ceramint.2020.05.240.
D.O. Moskovskikh, S. Vorotilo, A.S. Sedegov, K.V. Kuskov, K.V. Bardasova, P.V. Kiryukhantsev-korneev, M. Zhukovskyi, and A.S. Mukasyan: Ceram. Int., 2020, vol. 46(11), pp. 19008–14. https://doi.org/10.1016/j.ceramint.2020.04.230.
S. Ning, T. Wen, B. Ye, and Y. Chu: J. Am. Ceram. Soc., 2020, vol. 103(3), pp. 2244–51. https://doi.org/10.1111/jace.16896}.
C. Suryanarayana: Mater. Res. Lett., 2022, vol. 10(10), pp. 619–47. https://doi.org/10.1080/21663831.2022.2075243.
J. Rodríguez-Carvajal: Physica B, 1993, vol. 192(1), pp. 55–69. https://doi.org/10.1016/0921-4526(93)90108-I.
G.K. Williamson and W.H. Hall: Acta Metall., 1953, vol. 1(1), pp. 22–31. https://doi.org/10.1016/0001-6160(53)90006-6.
J. Kansy: Nucl. Instrum. Methods Phys. Res. Sect. A, 1996, vol. 374(2), pp. 235–44. https://doi.org/10.1016/0168-9002(96)00075-7.
K. Nakamura and M. Yashima: Mater. Sci. Eng. B, 2008, vol. 148(1), pp. 69–72. https://doi.org/10.1016/j.mseb.2007.09.040.
A.S. Kurlov and A.I. Gusev: Inorg. Mater., 2006, vol. 42(2), pp. 121–27. https://doi.org/10.1134/S0020168506020051.
H. Okamoto: J. Phase Equilib. Diffus., 2008, vol. 29(6), pp. 543–44. https://doi.org/10.1007/s11669-008-9396-7.
L. Feng, W.G. Fahrenholtz, G.E. Hilmas, and Y. Zhou: Scripta Mater., 2019, vol. 162, pp. 90–93. https://doi.org/10.1016/j.scriptamat.2018.10.049.
C.M. Rost, E. Sachet, T. Borman, A. Moballegh, E.C. Dickey, D. Hou, J.L. Jones, S. Curtarolo, and J.-P. Maria: Nat. Commun., 2015, vol. 6(1), p. 8485. https://doi.org/10.1038/ncomms9485.
J.R.D.S. Politi, F. Vines, J.A. Rodriguez, and F. Illas: Phys. Chem. Chem. Phys., 2013, vol. 15, pp. 12617–25. https://doi.org/10.1039/C3CP51389K.
V.G. Zavodinsky: Int. J. Refract. Met. Hard Mater., 2010, vol. 28(3), pp. 446–50. https://doi.org/10.1016/j.ijrmhm.2010.02.003.
B.H. Toby: Powder Diffr., 2006, vol. 21(1), pp. 67–70. https://doi.org/10.1154/1.2179804.
R. Idczak, W. Nowak, M. Babij, and V.H. Tran: Physica C, 2021, vol. 590, p. 1353945. https://doi.org/10.1016/j.physc.2021.1353945.
R.M. Nieminen, and M.J. Manninen, in: Positrons in Imperfect Solids: Theory, P. Hautojärvi, (ed.), Springer, Berlin, pp. 145–95, 1979. https://doi.org/10.1007/978-3-642-81316-0_4
A.A. Rempel, A.A. Valeeva, A.S. Kurlov, G. Klinser, and W. Sprengel: Mendeleev Commun., 2019, vol. 29(5), pp. 486–88. https://doi.org/10.1016/j.mencom.2019.09.002.
M.J. Puska, M. Sob, G. Brauer, and T. Korhonen: J. Phys. IV France, 1995, vol. 05, pp. 1–351142. https://doi.org/10.1051/jp4:1995115.
J.M.C. Robles, E. Ogando, and F. Plazaola: J. Phys., 2007, vol. 19(17), 176222https://doi.org/10.1088/0953-8984/19/17/176222.
J.-G. Choi: Appl. Catal. A, 1999, vol. 184(2), pp. 189–201. https://doi.org/10.1016/S0926-860X(99)00109-X.
M. Jalaly, F.J. Gotor, and M.J. Sayagués: Int. J. Refract. Met. Hard Mater., 2019, vol. 79, pp. 177–84. https://doi.org/10.1016/j.ijrmhm.2018.12.011.
A. Vladescu, I. Titorencu, Y. Dekhtyar, V. Jinga, V. Pruna, M. Balaceanu, M. Dinu, I. Pana, V. Vendina, and M. Braic: PLoS ONE, 2016, vol. 11(8), pp. 1–18. https://doi.org/10.1371/journal.pone.0161151.
K. Kowalski: In situ xps investigation of the baking effect on the surface oxide structure formed on niobium sheets used for superconducting rf cavity production. in Proc. of the 11th Workshop on RF Superconductivity, Travemünde, Germany, 2003
C. Wan, Y.N. Regmi, and B.M. Leonard: Angew. Chem. Int. Ed., 2014, vol. 53(25), pp. 6407–10. https://doi.org/10.1002/anie.201402998.
S. Li, Y. Zhang, Y. Han, F. Lv, B. Liu, and L. Huo: Appl. Surf. Sci., 2022, vol. 600, 154134https://doi.org/10.1016/j.apsusc.2022.154134.
P.V. Krasovskii, O.S. Malinovskaya, A.V. Samokhin, Y.V. Blagoveshchenskiy, V.A. Kazakov, and A.A. Ashmarin: Appl. Surf. Sci., 2015, vol. 339, pp. 46–54. https://doi.org/10.1016/j.apsusc.2015.02.152.
C. Lopes, N.M.G. Parreira, S. Carvalho, A. Cavaleiro, J.P. Riviére, E. Le Bourhis, and F. Vaz: Surf. Coat. Technol., 2007, vol. 201(16), pp. 7180–86. https://doi.org/10.1016/j.surfcoat.2007.01.025.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, 1980.
H. Lun, Y. Zeng, X. Xiong, Z. Ye, Z. Zhang, X. Li, H. Chen, and Y. Liu: J. Adv. Ceram., 2021, vol. 10(4), pp. 741–57. https://doi.org/10.1007/s40145-021-0469-y.
L.B. Dubrovskaya and S.Z. Nazarova: Phys. Status Solidi A, 1984, vol. 83(1), pp. 11–38. https://doi.org/10.1002/pssa.2210830102.
H. Bittner and H. Goretzki: Monatshefte für Chemie und verwandte Teile anderer Wissenschaften, 1962, vol. 93(5), pp. 1000–04. https://doi.org/10.1007/BF00905899.
L. Toth: Transition Metal Carbides and Nitrides, Elsevier Science, Amsterdam, 2014.
A.I. Gusev, A.A. Rempel, and V.N. Lipatnikov: Magnetic susceptibility and atomic ordering in tantalum carbide. Phys. Status Solidi A, 1988, vol. 106(2), pp. 459–66. https://doi.org/10.1002/pssa.2211060216.
G. Subhashree, S. Sankar, and R. Krithiga: Zeitschrift für Naturforschung A, 2015, vol. 70(9), pp. 721–28. https://doi.org/10.1515/zna-2015-0126.
N.J. Szymanski, I. Khatri, J.G. Amar, D. Gall, and S.V. Khare: J. Mater. Chem. C, 2019, vol. 7, pp. 12619–32. https://doi.org/10.1039/C9TC03793D.
R.H. Willens and E. Buehler: Appl. Phys. Lett., 1965, vol. 7(1), pp. 25–26. https://doi.org/10.1063/1.1754239.
P.B. Allen and M.L. Cohen: Phys. Rev. Lett., 1972, vol. 29, pp. 1593–96. https://doi.org/10.1103/PhysRevLett.29.1593.
P. Koželj, S. Vrtnik, A. Jelen, S. Jazbec, Z. Jagličić, S. Maiti, M. Feuerbacher, W. Steurer, and J. Dolinšek: Phys. Rev. Lett., 2014, vol. 113, 107001https://doi.org/10.1103/PhysRevLett.113.107001.
P. Sobota, R. Topolnicki, T. Ossowski, T. Pikula, A. Pikul, and R. Idczak: Phys. Rev. B, 2022, vol. 106, 184512https://doi.org/10.1103/PhysRevB.106.184512.
R.-Z. Zhang and M.J. Reece: J. Mater. Chem. A, 2019, vol. 7, pp. 22148–62. https://doi.org/10.1039/C9TA05698J.
Q. Zhu, G. Xiao, Y. Cui, W. Yang, S. Wu, G.-H. Cao, and Z. Ren: Scripta Mater., 2022, vol. 210, 114464https://doi.org/10.1016/j.scriptamat.2021.114464.
Q. Zhu, G. Xiao, Y. Cui, W. Yang, S. Song, G.-H. Cao, and Z. Ren: J. Alloy Compd., 2022, vol. 909, 164700https://doi.org/10.1016/j.jallcom.2022.164700.
L.B. Mendelsohn, F. Biggs, and J.B. Mann: Phys. Rev. A, 1970, vol. 2, pp. 1130–34. https://doi.org/10.1103/PhysRevA.2.1130.
D.J. Safarik, R.B. Schwarz, and M.F. Hundley: Phys. Rev. Lett., 2006, vol. 96, 195902https://doi.org/10.1103/PhysRevLett.96.195902.
C.A. Martin: J. Phys., 1991, vol. 3(32), p. 5967. https://doi.org/10.1088/0953-8984/3/32/005.
E.S.R. Gopal: Specific Heats at Low Temperatures, Plenum Press, New York, 1966. https://doi.org/10.1007/978-1-4684-9081-7.
F.I. Ajami and R.K. MacCrone: J. Less Common Met., 1974, vol. 38(2), pp. 101–10. https://doi.org/10.1016/0022-5088(74)90053-8.
X.-G. Lu, M. Selleby, and B. Sundman: Acta Mater., 2007, vol. 55(4), pp. 1215–26. https://doi.org/10.1016/j.actamat.2006.05.054.
Y. Liu, Y. Jiang, R. Zhou, and J. Feng: J. Alloy Compd., 2014, vol. 582, pp. 500–04. https://doi.org/10.1016/j.jallcom.2013.08.045.
Acknowledgments
This work was financed by the National Science Center (Poland) under the OPUS 20 Project No. 2020/39/B/ST5/01782. For the purpose of Open Access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Idczak, R., Sobota, P., Tran, L.M. et al. New Route to Synthesize High-Entropy Carbide Powders by Mechanical Alloying. Metall Mater Trans A 55, 523–536 (2024). https://doi.org/10.1007/s11661-023-07263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-07263-x