Abstract
In the present work, the dynamic recrystallization and microstructural evolution of the family of advanced high-strength steels Fe18MnxAl0.74C are studied, varying the aluminum content in 0, 3, 6, and 9 wt pct subjected to hot-forging treatments through three consecutive heating-deformation cycles. For characterization, X-ray diffraction (XRD), Mössbauer absorption spectroscopy (MAS), and electron backscattering diffraction (EBSD) were used. It was determined that for the steels under study, dynamic recrystallization occurs due to strain-induced boundary migration (SIBM) and is strongly influenced by the aluminum content of the alloy and its stacking failure energy (SFE), increasing that the aluminum content will generate greater nucleation sites, favoring the refinement of grains in the material and achieving a crystalline structure of random crystallographic orientation. The results are discussed throughout the article, allowing us to determine potential processing routes for advanced high-strength steels with predominantly plastic deformation mechanisms such as transformation-induced plasticity (TRIP), twinning-induced plasticity (TWIP), and microband-induced plasticity (MBIP).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Under the environmental commitment to reduce the impacts of greenhouse gas from the automotive and transportation industry, the development of lightweight alloys has been considered one of the most important solutions to this problem.[1] In pursuit of this objective, aluminum, magnesium, and high-strength steel have been used.[2] In the case of high-strength steels, Fe–Mn–Al–C steels have been presented as a main replacement candidate given their mechanical properties, ranging from a yield strength of 0.4 to 1.5 GPa, an ultimate tensile strength of 0.6 to 2.0 GPa, and an elongation of 30 to 140 pct,[3,4,5] with reduced density being one of its main physical characteristics, achieving decreases of 1.3 pct per 1 wt pct Al addition[6] with a maximum of 16 pct compared to the density of conventional steels.[7,8]
According to the chemical composition of this alloy system at room temperature, the following phases can be present: γ-austenite, α-ferrite, κ-carbides, ε-martensite, and some precipitates as \({\text{M}}_{3}{\text{C}}\) carbides, β-Mn, and \({\text{M}}_{5}\left({\text{Si}},{\text{Al}}\right).\) [9,10,11] The steels γ-austenite, duplex (γ-austenite + \(\alpha \)-ferrite), and γ-austenite + κ-carbides y triplex (γ-austenite + α-ferrite + κ-carbides) are the ones that have most intrigued numerous researchers due to their properties.[12,13,14,15,16,17,18,19] Numerous studies on γ-austenite steels are focused on the mechanism of strain hardening that occurs as a function of the stacking fault energy (SFE).[13,14,17,20,21,22,23,24,25,26,27,28,29,30,31,32]
However, the optimization of the mechanical properties is closely related to the processing of these steels, in which various authors have carried out studies on the hot workability of various Fe–Mn–Al–C steels (hot deformation behavior and processing map). An important parameter is the efficiency of power dissipation, which is defined as the efficiency with which energy dissipates through the change in microstructure during hot deformation. In this order of ideas, through processing maps, the efficiency of power dissipation, and instability criterion, the most favorable conditions for hot working have been determined for Fe–Mn–Al–C steels, where dynamic recrystallization phenomena occur.
Zambrano et al.[19] determined through a processing map the optimal conditions of temperature and strain rate for Fe21Mn11Al1.5C2Si. In a temperature range 900 °C to 1150 °C, with strain rates of 0.01, 0.1, and 1 s−1, and at to true strain of 0.7. Concluding that the optimal processing condition is at temperatures between 1000 °C and 1150 °C and strain rates between 0.01 and 0.1 s−1. Gwon et al.[26] studied the processing maps of Fe17Mn0.6C and Fe17Mn0.6C0.25V, in a temperature range of 800 °C to 1000 °C, with strain rate of 0.1, 0.5, 1, 5, and 10 s−1, and at to true strain of 0.5. Determining for V-free steel that the highest efficiency of power dissipation values is between 920 °C and 1000 °C at strain rates lower than 1 s−1, while for steel alloyed with V, they are between 850 °C and 1000 °C at strain rate greater than 10 s−1, suggesting that the addition of V makes dynamic recrystallization (XRD) processes more frequent. Liu et al.[33] studied the processing maps of Fe11Mn10Al0.9C in a temperature range of 800 °C to 1100 °C, with strain rates between 0.001 and 10 s−1 and true strains of 0.1, 0.2, 0.4, and 0.7. For all true strains, at strain rates between 0.001 and 0.03 s−1 and temperatures between 800 and 1000 °C, the highest values of efficiency of power dissipation are obtained. Similarly, Wu et al.[34] studied the processing maps of Fe26Mn8Al1C and Fe26Mn10Al1C in a temperature range of 850 °C to 1150 °C at strain rates in a range of 10−6 to 102 s−1 at a true strain of 0.5. For Fe26Mn8Al1C steel, the highest efficiency of power dissipation values is found at temperatures between 1000 °C and 1150 °C and strain rates of 10−6 to 10−4 s−1. On the other hand, for the case of Fe26Mn10Al1C steel, the optimal values are between 850 °C and 1050 °C at 10−6 s−1 and 900 °C to 1000 °C at 102 s−1.
Although these studies have been carried out at low strain rates, various studies on Fe–Mn–Al–C steels show that at high deformation rates of \(\dot{\varepsilon }=10\,{ \mathrm{s}}^{-1}\), greater grain refinement is evidenced in hot deformation by isothermal compression compared to lower strain rates,[34,35] which from the point of view, grain boundary engineering (GBE) is favorable. Additionally, there is evidence of the relationship between the strain rate, grain size, the fraction of recrystallized grains, and the generation of twins in various alloy systems, which shows that at strain rates of approximately \(\dot{\varepsilon }=10\,{ \mathrm{s}}^{-1}\), recrystallization processes are favored,[36,37,38,39,40,41,42] achieving a higher fraction of recrystallized grains, smaller grains size and, in some cases, the same fraction of ∑3 compared to low strain rate. Although these favorable results for high strain rates are not entirely clear, various authors[37,38,40,42,43,44] associate adiabatic temperature as one of the main factors that could influence dynamic recrystallization processes at high strain rates because this local increase in temperature generated by the high rates of plastic deformation favors the nucleation and migration of grain boundaries.
Previous studies show that the dynamic recrystallization phenomena for each Fe–Mn–Al–C steel are strongly influenced by the hot-processing parameters (temperature and strain rate). To the best of our knowledge, there is currently no detailed study of the microstructural evolution of Fe–Mn–Al–C steels with varying aluminum content that allows us to understand how this element affects dynamic recrystallization. The present research aims to present the behavior of Fe18MnxAl0.74C steels (x = 0, 3, 6, and 9) through a hot-forging treatment at 950 °C and a strain rate of 10 s−1 in a Wagner 500-ton screw hydraulic press. For this purpose, X-ray diffraction (XRD), Mössbauer absorption spectroscopy (MAS), and electron backscatter diffraction (EBSD) were used to study the structural and microstructural evolution when varying the aluminum content.
2 Methodology
The Fe–Mn–Al–C steels were melted in an induction furnace starting from AISI 1020 and 1045, high- and low-carbon ferro-manganese, ferro-silicon, ferro-molybdenum, copper, and aluminum with purities higher than 99 pct. Chemical characterization was made using optical emission spectroscopy (OES) and X-ray fluorescence (XRF) techniques. For the calculation of the SFE, the thermodynamic model of Zambrano et al.[45] was used assuming a temperature of 25 °C and that the microstructure of the steels was totally austenite. The phase diagrams in equilibrium condition were made in the JMatPro software. Table I presents the results of the chemical analysis and calculated SFE values.
The samples in the “as-cast” condition were cut in dimensions of 100 mm × 22 mm × 15 mm and forged in a Wagner 500-ton screw hydraulic press, at a temperature of 950 °C and strain rate of 10 s−1, achieving a total reduction of ~ 50 pct (cumulative true strain 0.91) through 3 reduction steps, each of 3 mm and finally, quenching in water (after the last reduction) to avoid any phase transformation. For the characterization of the microstructure of Fe–Mn–Al–C steels, X-ray diffraction (XRD), Mössbauer absorption spectroscopy (MAS), scanning electron microscopy (SEM-EDS), and backscatter electron diffraction (EBSD) were used.
Characterization by XRD was in an X'Pert PRO PANalytical diffractometer using various radiation sources for the generation of the diffractograms. For the Fe–Mn–Al–C alloys in the “as-cast” condition, copper radiation was used (Kα1 = 1.5406 Å and Kα2 = 1.5444 Å), and those treated hot forging were cobalt radiation (Kα1 = 1.78901 Å and Kα2 = 1.7929 Å). GSAS software[46] was used to refine the diffractograms, and LaB6 powder was used as a calibration sample; additionally, the samples were measured under bulk conditions.
MAS was performed in high-speed transmission mode (from − 12 to 12 mm/s) for samples with magnetic behavior and low-speed transmission mode (from − 5 to 5 mm/s) for paramagnetic samples, with a radioactive source of cobalt-57 (57Rh). The fit of these spectra was performed with MOSFIT software, using a thin sheet of α-Fe as a calibration sample. The presentation of these samples consisted of powder extracted from the solid pieces, for which an abrasive process at low speed with a diamond lime to avoid inducing phase transformations due to mechanical stress and to avoid contamination of the sample.
The semi-quantitative elemental analysis was performed on a JEOL JSM-6490LV scanning electron microscope (SEM). Due to the high variation in the measurements generated by C due to its low atomic weight, it was not taken into account in the determined values of pct by weight of the elemental analysis. For the characterization of electron backscatter diffraction (EBSD), INSPECT F50 SEM equipment was used. The samples were prepared using the conventional metallographic methodology, followed by a final polishing procedure carried out in a Minimet 1000 Buhler fine polishing machine with 0.04 µm colloidal silica for 3 hours and 20 minutes. SEM was used with an acceleration voltage of 20 kV at a working distance of 16 mm. The area scanned by EBSD was 460 µm × 485 µm and was analyzed with a separation step of 0.47 µm between each point. For the EBSD analysis that includes the phase map, the blue and red colors designate the austenite (γ) and ferrite (α) phases, respectively. The limits were classified as low-angle grain boundary limits (LAGBs) for the misorientation (θ) that satisfied 1 deg ≤ θ < 15 deg, while it was determined that the high-angle grain boundary limits (HAGBs) had greater or equal misorientation at 15 deg.[47,48] The twins, regardless of whether they were annealed or mechanical, they were classified with misorientation angles of 60 deg on the 〈111〉 axis[19,48] with a maximum deviation of 4 deg.
3 Results
3.1 Mössbauer Absorption Spectroscopy and X-ray Diffraction
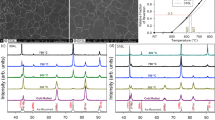
In Figures 1 and 2, the Mössbauer spectra of the Fe–Mn–Al–C samples are presented in “as-cast” conditions and hot forging treated, respectively.
According to the Mössbauer spectra, the behavior of the steels, both in “as-cast” and hot-forging conditions (Fe18Mn0Al, Fe18Mn3Al, and Fe18Mn6Al), is totally paramagnetic; that is a single-broad singlet is presented that corresponds to the austenite phase (f.c.c). In the case of Fe18Mn9Al steel, in addition to presenting the characteristic singlet of austenite (f.c.c), it also presents a magnetic contribution in the spectrum, which is associated with the ferrite phase (b.c.c).
The Mössbauer fit of these spectra was performed by 3 types of austenites: base austenite, solute-rich austenite (high C and Al), and solute-poor austenite (low C and Al). The reason for this, in addition to achieving a better fit compared to being fitted with a single austenite, lies mainly in the large width of the singlet of the Mössbauer spectra (Figures 1 and 2), which indicates that iron atoms have more than a chemical environment around it.[49] This is reported by Duran et al.[50] for Fe20Mn3Al0.7C and Fe20Mn6Al0.7C, indicating by Mössbauer parameter, isomer shift (IS), the presence of various austenites resulting from the spinodal decomposition process that occurs in this steels. Considering the information before, in the present investigation, various environments around the iron atom were considered due to the variation of the Mössbauer parameters, such as the isomer shift, which is a sensitive parameter to the atomic environments around iron with the alloy elements. An explanation of this is extended in Section IV–A.
Table II presents the Mössbauer parameters obtained from the fit of the spectra in Figures 1 and 2. For the case of the paramagnetic samples, the austenite phase (f.c.c) was fitted with three different singlets, each of which is characterized by a different isomer shifts, and for the case of the sample that also presents the ferrite phase (b.c.c), it is characterized by its mean hyperfine field value.
In Figures 3 and 4, the XRD results are presented for the Fe–Mn–Al–C samples in “as-cast” conditions and hot forging treated. The fit of the diffractograms in the “as-cast” condition as well as in the hot-forging condition was made with 3 types of austenites.
According to Figure 3, the diffractograms of the Fe18Mn0Al, Fe18Mn3Al, and Fe18Mn6Al samples correspond to the austenite phase (f.c.c). Furthermore, the sample Fe18Mn9Al, additionally diffraction in the characteristic planes of the austenite phase (f.c.c), also diffracted in the plane (110), which corresponds to ferrite (b.c.c), which leads to the conclusion together with the Mössbauer results (Figure 1(d)) that the Fe18Mn9Al sample is duplex steel. Additionally, this sample presents diffraction peaks in planes (210) and (220), which correspond to a phase formed between Al and Mn. These diffraction peaks are possibly due to the chemical segregation present in the dendritic condition since this generates phases rich in elements with a low melting point in the material.
In Figure 4, it is identified that the samples contain for the case of Fe18Mn0Al, Fe18Mn3Al, and Fe18Mn6Al a single phase corresponding to austenite (f.c.c); moreover, for the diffractograms of the Fe18Mn9Al alloys, as well appreciating the austenite phase (f.c.c), the presence of the ferrite phase (b.c.c) becomes evident, and these results coincide with the Mössbauer results for this sample (Figure 2(d)). Given the homogeneity condition generated by the hot-forging treatment, the Fe18Mn9Al sample no longer presents diffraction of the Al-Mn rich phase, which is present in the “as-cast” condition.
Table III shows the lattice parameter, pct weight of each phase, fit parameters, and density of the Fe–Mn–Al–C steels.
It is observed that the lattice parameter for the same steel under different conditions, “as-cast” and hot forging treated, presents a decrease, which indicates the structural ordering that occurs during processing. Additionally, the intensity peaks shift toward lower 2thetas angles due to the increase in the aluminum content in the solid solution of the Fe–Mn–Al–C alloys. Finally, it is possible to determine a trend in the decrease in the density of Fe–Mn–Al–C steels by increasing the aluminum content, which in the case of the steels under study is 3.35 pct for 18Mn3Al steel, 5.93 pct for 18Mn6Al, and 10.57 pct for 18Mn9Al compared to the sample without 18Mn0Al aluminum content, which indicates that the addition of 1 wt pct in Al reduces the density by approximately 1.21 pct, which coincides with Refrence 6.
3.2 Hot-Forging Treatment
Figure 5 shows the grain orientation spread (GOS) micrographs, corresponding to the samples under study. This type of micrograph allows us to know the distribution of the misorientation angles within and between the grains (crystals). This makes it possible to determine the current state of the grains, that is, if a grain has recently been recrystallized (low angles of misorientation) by the hot-forging treatment process or not (high angles of misorientation).[19,47,51,52,53]
Figure 6 shows the distribution of GOS values from the data in Figure 5. According to this, for Fe18Mn0Al about 46.8 pct of the grains correspond to misorientation between 0 and 3 deg, for the Fe18Mn3Al sample, this value corresponds to 36.6 pct, the Fe18Mn6Al sample 64 pct and for the Fe18Mn9Al sample 46 pct corresponds to the austenite phase and 15.3 pct to the ferrite. This indicates that most of the grains formed are recrystallized grains, as a result of the hot-forging treatment.
The GOS value distribution of Fig. 5. (a) Fe18Mn0Al, (b) Fe18Mn3Al, (c) Fe18Mn6Al, and (d) Fe18Mn9Al
Figure 7 shows the grain size distribution of the samples, in which it is possible to observe the effect of increasing the aluminum content on grain refinement. In the case of the Fe18Mn0Al sample, 45.2 pct corresponds to grain sizes between 0 and 20 µm, for the Fe18Mn3Al sample 60.39 pct, for Fe18Mn6Al 75.23 pct, and for the Fe18Mn9Al sample 71.69 pct corresponds to the austenite phase and 20.17 pct to the ferrite. Under the same hot-forging condition, post-recrystallization processes are taking place in the Fe18Mn0Al sample, since 54.8 pct of the grains has a grain size greater than 20 µm, meanwhile for Fe18Mn3Al, Fe18Mn6Al, and Fe18Mn9Al, this percentage is 39.61, 24.77, and 8.14 pct, respectively. This is explained in more detail in Section IV–A.
In Figure 8, crystallographic orientation maps of the hot-forging treated Fe–Mn–Al–C samples are presented. The microstructure of the material does not result in preferential orientation. In contrast, the arrangement of the grains in front of the crystallographic directions they grew was completely random.
In Figure 9, the contour inverse pole figure (IPF) of the samples in Figure 8 is presented in a semi-quantitative manner. These contour maps relate the tonality of the lines, with the predominance of the crystallographic orientation of the grains. Red tones indicate a maximum, going from orange to yellow tones, from yellow to green, until reaching a minimum value corresponding to blue tones.
IPF and value contour in [001] orientation of hot-forging treated Fe–Mn–Al–C steels. The results obtained based on Fig. 8 for (a) Fe18Mn0Al, (b) Fe18Mn3Al, (c) Fe18Mn6Al, and (d) Fe18Mn9Al
It is confirmed that the crystallographic directions within the material are completely random. Observing that the material internally, after the forging process, does not acquire a preferential orientation due to the hot-forging treatment.
In Figure 10, the phase map of the hot-forging treated samples is presented. Blue corresponds to austenite (f.c.c), and red corresponds to ferrite (b.c.c). The increase in aluminum content in the Fe–Mn–Al–C alloy system favors the formation of ferrite, with 21 pct ferrite for Fe18Mn9Al.
Figure 11 shows the grain boundary maps with annealing twins ∑3 and length fraction of annealing twins ∑3,[54] generated by the hot-forging treatment. In this case, it is observed that the variation in aluminum increases the fraction of the twins, which is directly related to the increase in dynamic recrystallization (DRX). To determine the length fraction of annealing twins ∑3 (Figure 11(e)), only the grain boundaries of the austenitic phase were taken into account.
3.3 Microsegregation in Fe–Mn–Al–C After Hot-Forging Treatment
Figure 12 shows the phase diagrams under equilibrium conditions for Fe–Mn–Al–C steels, and in this figure, we can see the precipitates that can be formed depending on the alloying elements and the hot-working temperature. According to the hot-forging temperature, at 950 °C, there is the possibility of formation of M2 (C,N) precipitates for Fe18Mn0Al and Fe18Mn3Al, while for Fe18Mn6Al and Fe18Mn9Al, the formation of precipitates of type M6C.
The equilibrium phase diagrams of (a) Fe18Mn0Al, (b) Fe18Mn3Al, (c) Fe18Mn6Al, and (d) Fe18Mn9Al. According to the chemical composition of Table I
Through the semi-quantitative SEM-EDS analysis, elemental analysis of precipitates and/or chemical segregation generated in the steels after the hot-forging treatment was made. These results are presented in Figure 13.
According to Figure 13, the main elements present in the precipitates are P, Si, Mo, S, Al, Cu, Fe, and Mn, which can form intermetallic compounds such as MnS, Al2O3, M2, and M6C carbides (Figure 12), where M can be elements such as Mo, Fe, Si, and Mn, present in steels. Precipitates enriched in these elements have been reported by Liu et al.[28] in Fe18.22Mn0.002Al0.61C and Fe17.7Mn0.75Al0.61C steels. Also, Durán et al.[50] report for the steels Fe20.11Mn3.07Al0.71C1.5Mo1.54Si0.77Cu and Fe23.4Mn6.6Al0.69C1.52Mo1.33Si0.684Cu, and the formation of precipitates enriched in Al, O, Si, P, Cu, and Mo after forging treatment at 950 °C.
4 Discussion
4.1 Mössbauer Absorption Spectroscopy and X-ray Diffraction
Given that Mössbauer spectroscopy has an extremely fine resolution in terms of energy and can detect even subtle changes in the nuclear environment of atoms, it is widely used for its ease in measuring hyperfine interactions (electrical and magnetic) between the nuclei of Mössbauer atoms such as iron and its closest chemical environment, which makes this technique suitable for the characterization of materials composed of iron. The Mössbauer spectra obtained using this technique (Figures 1 and 2) can provide information on the level of order–disorder at the structural level because it is capable of differentiating the different types of iron atoms according to their respective environments of the crystalline structure and the oxidation state in iron compounds.[55,56,57,58] Additionally, by obtaining information on the hyperfine interactions of Mössbauer atoms and being complemented with an additional technique such as X-ray diffraction (XRD), it is possible to identify crystalline structures present in iron alloys. In the particular case of this study, through the Mössbauer parameters, different types of austenites can be identified according to whether they are poor or rich with elements, such as Al, Mn, Mo, and Si, different from those that would have an austenitic structure made up of iron and carbon only.
It is well known that solid metallic solutions are not ideal from the point of view of chemical homogeneity, since there are some preferences in the closest neighborhoods of some elements for others.[55,59,60,61] On the other hand, in Fe–Mn–Al–C steels, the spinodal reaction can occur according to the following sequence: \(\gamma \to {\gamma }^{\prime}+{\gamma }^{\prime\prime}\to {\gamma }^{\prime}+{L1}_{2}\left(SRO\right)\to {\gamma }^{\prime}+{k}^{\prime}\).[62] This reaction causes the high-temperature decomposition of austenite into two types of austenites: solute-poor austenite (low concentration of C and Al atoms) and solute-rich austenite (high concentration of C and Al atoms). The second step of the reaction occurs as a result of quenching or cooling air from temperatures above 900 °C. Finally, the last step of the spinodal decomposition reaction in Fe–Mn–Al–C steels is known to produce \(k\)-carbides as a result of either slow cooling during a thermomechanical treatment,[19] or it is in solid solution, and it can be promoted by an aging treatment, which occurs with relatively prolonged maintenance at temperatures between 500 °C and 700 °C.[4,62] Given the methodology of hot-forging treatments and the chemical composition of the Fe–Mn–Al–C steels, the last step of the spinodal reaction will not occur, which corresponds to the formation of \(k\)-carbides; that is, the austenite will decompose into solute-poor austenite and solute-rich austenite, generating an incomplete spinodal decomposition.
According to Table II, the isomer shift related to the Fe18Mn0Al, Fe18Mn3Al, and Fe18Mn6Al samples of the different austenites used for the fit does not vary considerably between the “as-cast” and hot-forging treated conditions. The importance of the isomer shift lies in the information that it provides on the distribution of the external s and d electrons of the resonant atom. Finally, these solute-poor and solute-rich austenites derive from atomic interactions and electron exchange between solute atoms, with Fe atoms in the f.c.c. In the case of Fe18Mn9Al steel, which is a duplex phase, generates a greater degree of disorder at the atomic level, finding greater differences between the isomer shifts compared to the austenitic samples. This is corroborated by observing the mean hyperfine field of the ferrite present in the Fe18Mn9Al, which corresponds to highly disordered ferrite, since the hyperfine field of ferrite (pure iron) corresponds to 330 T.[49]
4.2 Hot-Forging Treatment
According to the results of Section III–B, it is possible to observe the variation of the microstructure of the Fe–Mn–Al–C steels as a function of the aluminum content, which is related to the variation of the SFE. The morphological characteristics exhibited in Figure 5, of small grains with low angles of misorientation, surrounding large grains with higher angles of misorientation, are clear proof of the dynamic recrystallization by strain-induced boundary migration (SIBM). The explanation for the refinement of the microstructure through hot-forging treatments is based on the following: the lower aluminum content (low SFE Fe–Mn–Al–C steels) favors the accelerated generation of differences in dislocation densities between grain boundary (high \(\Delta E\)), this increases the driving force of the SIBM, generating new grains with a low degree of misorientation (low stored dislocation energy).[63,64] These previously formed grains grow at the expense of others by the consuming the remaining dislocations, leading to a growth of the size of grain (post-recrystallization) this is evidenced in the evolution of the distribution of grain sizes in Figure 7. On the other hand, the samples with higher aluminum content (medium or high SFE Fe–Mn–Al–C steels), have more difficulty generating sufficient differences in dislocation densities between grain boundaries (low \(\Delta E\)), facilitating the creation of multiple SIBM,[37,65] due to the low-density level of dislocations, the grains formed will not present post-recrystallization. For Fe18Mn9Al duplex steel, the grain refinement ends up being the joint action generated by multiple SIBM and the nucleation dynamics of a second phase (ferrite).[66,67,68] Due to grain boundaries, migration by SIBM is promoted the “growth accident.” in which a coherent twin boundary (Σ3n) is formed at a migratory grain boundary due to stacking error during growth under energetically favorable conditions.[69,70,71,72] The results presented in Figures 6, 7, and 11(e) show that the samples with finer grain distributions that also have low GOS distributions, present an increase in length fraction of Σ3, which indicates that the formation of annealing twins is favored by DRX processes. These results agree with References 23, 38, 73, in which there is a linear relationship between the formation of new grains and the formation of Σ3 twins.
5 Conclusions
In this research, the effect of the aluminum content in the Fe18MnxAl0.74C family of steels on dynamic recrystallization and microstructural evolution was studied when these steels were subjected to hot-forging treatments at an industrial level. The main conclusions of this work are presented as follows:
-
The presence of three different austenites was determined: solute-rich, solute-poor, and base austenite. The main hypothesis of this result is the occurrence of spinodal decomposition.
-
It was determined that the recrystallization mechanism for the study steels is of the strain-induced boundary migration (SIBM). The increase in the aluminum content favored a refinement of the grain.
-
It was determined that the appearance of ferrite in steel with a higher aluminum content favored grain refinement since this phase favors the heterogeneous nucleation of new grains, limiting the growth of adjacent austenite grains.
-
It was determined that the increase in annealing twins with increasing aluminum content is more related to the DRX processes than to the increase in SFE.
References
X. Cui, H. Zhang, S. Wang, L. Zhang, and J. Ko: Mater. Des., 2011, vol. 32, pp. 815–21.
K. Kawajiri, M. Kobayashi, and K. Sakamoto: J. Clean. Prod., 2020, vol. 253, pp. 119805–13.
O.A. Zambrano: J. Mater. Sci., 2018, vol. 53, pp. 14003–62.
S. Chen, R. Rana, A. Halder, and R.K. Ray: Prog. Mater. Sci., 2017, vol. 89, pp. 345–91.
B.C. De Cooman, L. Chen, H. Soo Kim, Y. Estrin, S.K. Kim, and H. Voswinckel: Microstructure and Texture in Steels, Springer, New York, 2009, pp. 165–83.
G. Frommeyer and U. Brüx: Steel Res. Int., 2006, vol. 77, pp. 627–33.
Y. Sutou, N. Kamiya, R. Umino, I. Ohnuma, and K. Ishida: ISIJ Int., 2010, vol. 50, pp. 893–99.
G. Frommeyer, E.J. Drewes, and B. Engl: Rev. Métall., 2000, vol. 97, pp. 1245–53.
H. Kim, D.-W. Suh, and N.J. Kim: Sci. Technol. Adv. Mater., 2013, vol. 14, pp. 14205–116.
Y.-U. Heo, Y.-Y. Song, S.-J. Park, H.K.D.H. Bhadeshia, and D.-W. Suh: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 1731–35.
A. Sarkar, S. Sanyal, T.K. Bandyopadhyay, and S. Mandal: Mater. Sci. Eng. A, 2017, vol. 703, pp. 205–13.
O.A. Zambrano, Y. Aguilar, J. Valdés, S.A. Rodríguez, and J.J. Coronado: Wear, 2016, vol. 348–349, pp. 61–68.
A. Kovalev, A. Jahn, A. Weiß, and P.R. Scheller: Steel Res Int., 2011, vol. 82, pp. 45–50.
L. Zhang, R. Song, C. Zhao, and F. Yang: Mater. Sci. Eng. A, 2015, vol. 640, pp. 225–34.
J. Moon, S.-J. Park, J.H. Jang, T.-H. Lee, C.-H. Lee, H.-U. Hong, D.-W. Suh, S.H. Kim, H.N. Han, and B.H. Lee: Scripta Mater., 2017, vol. 127, pp. 97–101.
A. Soulami, K.S. Choi, Y.F. Shen, W.N. Liu, X. Sun, and M.A. Khaleel: Mater. Sci. Eng. A, 2011, vol. 528, pp. 1402–08.
J.D. Yoo and K.-T. Park: Mater. Sci. Eng. A, 2008, vol. 496, pp. 417–24.
O.A. Zambrano, J. Valdés, L.A. Rodriguez, D. Reyes, E. Snoeck, S.A. Rodríguez, and J.J. Coronado: Tribol. Int., 2019, vol. 135, pp. 421–31.
O.A. Zambrano, J. Valdés, Y. Aguilar, J.J. Coronado, S.A. Rodríguez, and R.E. Logé: Mater. Sci. Eng. A, 2017, vol. 689, pp. 269–85.
B.-G. Zhang, X.-M. Zhang, and H.-T. Liu: Mater. Sci. Eng. A, 2020, vol. 793, pp. 139289–301.
J.H. Choi, M.C. Jo, H. Lee, A. Zargaran, T. Song, S.S. Sohn, N.J. Kim, and S. Lee: Acta Mater., 2019, vol. 166, pp. 246–60.
S. Lee, Y. Estrin, and B.C. De Cooman: Metall. Mater. Trans. A, 2014, vol. 45, pp. 717–30.
D.P. Field, L.T. Bradford, M.M. Nowell, and T.M. Lillo: Acta Mater., 2007, vol. 55, pp. 4233–41.
U. Brüx, G. Frommeyer, O. Grässel, L.W. Meyer, and A. Weise: Steel Res. Int., 2002, vol. 73, pp. 294–98.
O. Grässel, L. Krüger, G. Frommeyer, and L.W. Meyer: Int. J. Plast., 2000, vol. 16, pp. 1391–409.
H. Gwon, S. Shin, J. Jeon, T. Song, S. Kim, and B.C. De Cooman: Met. Mater. Int., 2019, vol. 25, pp. 594–605.
K.K. Anand, B. Mahato, C. Haase, A. Kumar, and S.G. Chowdhury: Mater. Sci. Eng. A, 2018, vol. 711, pp. 69–77.
H. Liu, J. Liu, Wu. Bowei, Y. Shen, Y. He, H. Ding, and Su. Xiaofeng: Mater. Sci. Eng. A, 2017, vol. 708, pp. 360–74.
E. Welsch, D. Ponge, S.M.H. Haghighat, S. Sandlöbes, P. Choi, M. Herbig, S. Zaefferer, and D. Raabe: Acta Mater., 2016, vol. 116, pp. 188–99.
I. Gutierrez-Urrutia and D. Raabe: Acta Mater., 2011, vol. 59, pp. 6449–62.
K.-T. Park, K.G. Jin, S.H. Han, S.W. Hwang, K. Choi, and C.S. Lee: Mater. Sci. Eng. A, 2010, vol. 527, pp. 3651–61.
J.A. Castañeda, O.A. Zambrano, G.A. Alcázar, S.A. Rodríguez, and J.J. Coronado: Metals, 2021, vol. 11, pp. 1701–21.
D. Liu, H. Ding, M. Cai, and D. Han: J. Mater. Eng. Perform., 2019, vol. 28, pp. 5116–26.
Wu. Zhiqiang, Y. Tang, W. Chen, Lu. Liwei, E. Li, Z. Li, and H. Ding: Vacuum, 2019, vol. 159, pp. 447–55.
Y. Chen, X.-M. Zhang, Z.-H. Cai, H. Ding, M.-M. Pan, and H.-S. Li: J. Mater. Eng. Perform., 2020, vol. 29, pp. 5503–14.
S. Mandal, A.K. Bhaduri, and V.S. Sarma: Materials Science Forum, Trans Tech Publ, Zurich, 2012, pp. 714–17.
S. Mandal, M. Jayalakshmi, A.K. Bhaduri, and V.S. Sarma: Metall. Mater. Trans. A, 2014, vol. 45A, pp. 5645–56.
D. Jia, W. Sun, Xu. Dongsheng, Yu. Lianxu, X. Xin, W. Zhang, and F. Qi: J. Alloys Compd., 2019, vol. 787, pp. 196–205.
X. Wang, D. Wang, J. Jin, and J. Li: Mater. Sci. Eng. A, 2019, vol. 761, pp. 138044–57.
H. Zhang, K. Zhang, H. Zhou, Lu. Zhen, C. Zhao, and X. Yang: Mater. Des., 2015, vol. 80, pp. 51–62.
Y. Cao, H. Di, J. Zhang, J. Zhang, T. Ma, and R.D.K. Misra: Mater. Sci. Eng. A, 2013, vol. 585, pp. 71–85.
M.A. Meyers, U.R. Andrade, and A.H. Chokshi: Metall. Mater. Trans. A, 1995, vol. 26A, pp. 2881–93.
Y. Liu, Y. Huang, M. Guan, X. Meng, and Y. Xie: Metall. Mater. Trans. A, 2020, vol. 51A, pp. 3349–53.
S.S.S. Kumar, T. Raghu, P.P. Bhattacharjee, G.A. Rao, and U. Borah: J. Alloys Compd., 2016, vol. 681, pp. 28–42.
O.A. Zambrano: J. Eng. Mater. Technol., 2016, vol. 138, pp. 1–9.
A.C. Larson and R.B. Von Dreele, In Los Alamos National Laboratory, (1994), p 231.
O.A. Zambrano and R.E. Logé: Mater. Charact., 2019, vol. 152, pp. 151–61.
O.A. Zambrano, G. Tressia, and R.M. Souza: Eng. Fail. Anal., 2020, vol. 115, pp. 104621–45.
P. Gütlich, E. Bill, and A.X. Trautwein: Mössbauer spectroscopy and transition metal chemistry: fundamentals and applications, Springer, New York, 2010.
J.F. Durán, G.A. Pérez, J.S. Rodríguez, Y. Aguilar, R.E. Logé, and O.A. Zambrano: Metall. Mater. Trans. A, 2021, vol. 52, pp. 4785–99.
T. Sakai, A. Belyakov, R. Kaibyshev, H. Miura, and J.J. Jonas: Prog. Mater Sci., 2014, vol. 60, pp. 130–207.
B. Xie, Yu. Hao, T. Sheng, Y. Xiong, Y. Ning, and M.W. Fu: J. Alloys Compd., 2019, vol. 803, pp. 16–29.
S.-C. Chen, C.-Y. Huang, Y.-T. Wang, and H.-W. Yen: Mater. Des., 2017, vol. 134, pp. 434–45.
W. Wang, F. Brisset, A.L. Helbert, D. Solas, I. Drouelle, M.H. Mathon, and T. Baudin: Mater. Sci. Eng. A, 2014, vol. 589, pp. 112–18.
V.G. Gavriljuk, B.D. Shanina, and H. Berns: Acta Mater., 2000, vol. 48, pp. 3879–93.
J.M. Greneche: J. Non-Cryst. Solids, 2001, vol. 287, pp. 37–44.
V.V. Ovchinnikov: Mössbauer Analysis of the Atomic and Magnetic Structure of Alloys, Cambridge Int Science Publishing, Cambridge, 2006.
M. Filatov: Coord. Chem. Rev., 2009, vol. 253, pp. 594–605.
T. Ericsson and J.B. Cohen: Acta Crystallogr. Sect. A, 1971, vol. 27, pp. 97–109.
K.C. Russel and D.F. Smith: in Physical Metallurgy of Controlled Expansion Invar-Type Alloys. Proceedings, K.C. Russel, D.F. Smith, eds., TMS, Warrendale, 1990, 331 p. ISBN 0-87339-099-7.
C.R. Houska and B.L. Averbach: J. Phys. Chem. Solids, 1962, vol. 23, pp. 1763–69.
W.-C. Cheng, C.-Y. Cheng, C.-W. Hsu, and D.E. Laughlin: Mater. Sci. Eng. A, 2015, vol. 642, pp. 128–35.
F.J. Humphreys and M. Hatherly: Recrystallization and Related Annealing Phenomena, 3rd ed. Elsevier, New York, 2017.
D.E. Laughlin and K. Hono: Physical Metallurgy, Newnes, Oxford, 2014.
J.E. Bailey and P.B. Hirsch: Proc. R. Soc. Lond. Ser A, 1962, vol. 267, pp. 11–13.
I. Ohnuma, K. Ishida, and T. Nishizawa: Philos. Mag. A, 1999, vol. 79, pp. 1131–44.
J.H. Park, Y. Tomota, and M.Y. Wey: Mater. Sci. Technol., 2002, vol. 18, pp. 1517–23.
R.W. Balluffi, S.M. Allen, and W.C. Carter: Kinetics of Materials, Wiley, New York, 2005.
C.S. Pande, M.A. Imam, and B.B. Rath: Metall. Mater. Trans. A, 1990, vol. 21A, pp. 2891–96.
H. Gleiter: Acta Metall., 1969, vol. 17, pp. 1421–28.
K.-H. Song, Y.B. Chun, and S.-K. Hwang: Mater. Sci. Eng. A, 2007, vol. 454, pp. 629–36.
S. Mahajan, C.S. Pande, M.A. Imam, and B.B. Rath: Acta Mater., 1997, vol. 45, pp. 2633–38.
X. Yang, P. Wang, and M. Huang: Mater. Sci. Eng. A, 2022, vol. 833, pp. 142532–41.
Acknowledgments
The authors would like to Colciencias (Colombian Agency), Organización Hercules, and Universidad del Valle for financial support under Project No. 1106-715-51419. Additionally, Universidad del Valle would like to receive financial support under project 71108 of internal convocation 105-2015. Oscar Zambrano would like to acknowledge the National Research Council Canada for the support. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied.
Funding
Open access funding provided by National Research Council Canada.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Missing Open Access funding information has been added in the Funding Note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez, J.S., Duran, J.F., Aguilar, Y. et al. Effect of Aluminum Content on the Dynamic Recrystallization of Fe18MnxAl0.74C Steels During Hot-Forging Treatments. Metall Mater Trans A 53, 2961–2976 (2022). https://doi.org/10.1007/s11661-022-06717-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-022-06717-y