Abstract
A model for the description of precipitation kinetics is presented and applied to experimental data for the evolution of the particle-size distribution in a dilute model system (Cu-Co) upon isothermal annealing at different temperatures. For coupling nucleation kinetics and growth kinetics, the model includes a recently proposed inverse evaluation method for consistent and numerically efficient evaluation of the thermodynamics of both nucleation and growth. Using the experimental data for the evolution of the particle-size distributions, obtained at least at two different temperatures, as a reference, unique and physically reasonable values for, at least, the interface energy and the activation energies for nucleation and growth can be obtained. The sensitivity of the kinetic model fitting to precise description of the thermodynamics of the particle–matrix system and the inferiority of kinetic model fitting to average data, such as data for the mean particle radius, have been highlighted.










Similar content being viewed by others
Notes
In extreme cases, the unphysical scenario can emerge that a particle once generated by nucleation is inherently unstable with respect to growth and, in this flawed model scenario, immediately begins to dissolve.
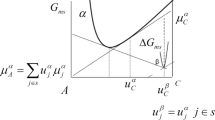
In the present work, the Gibbs energy is used to describe the energy content change of the system considered because of experimental control of \(p\) and \(T\).
Lattice parameters \(a^{j}\) and atomic volumes \(V^{j}\) of the phases \(j = \upalpha ,\,\upbeta \) are taken as being composition-independent.
in case of a binary system; for multinary systems cf. Reference 1.
i.e., a description of the chemical Gibbs energies of \(\upalpha \) and \(\upbeta \), or of the corresponding activity coefficients, as function of alloy composition and temperature
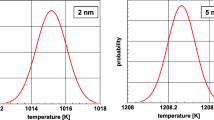
As compared to a representation of the data as histograms, the KDE approach avoids classification of the PSD into fixed, discrete particle-size classes.
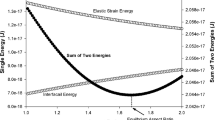
This is in particular caused by the different dependency of the size relation \(r(\Delta g_{\text{c}}(x^{\upalpha }), \gamma , \Delta g_{\text{el}})\) on \(\gamma \) and \(\Delta g_{\text{el}}\) (Eq. [6]).
This, of course, can be ascribed to the experimentally cumbersome determination of the evolution of the PSD as function of annealing time and temperature by using direct imaging techniques (as employed in the present case) or by its extraction from primary experimental data (e.g., in case of scattering experiments).[35]
In case of the nucleation rate, changes in \(D_{\text{N,0}}\) also affect the time lag \(\tau \) (with \(\exp ( - \tau / t)\) varying between 0 and 1); the nucleation rate thus does not scale exactly linearly with \(D_{\text{N,0}}\).
A variation of both activation energy and corresponding pre-factor is numerically underdetermined at constant \(T\).
Indeed, even with the full flexibility of the kinetic model provided by three fit parameters, it was not possible to achieve a model description for the experimental data at T = 748 K (475 °C) of a quality similar to that achieved at the higher temperatures. As a consequence, application of the model to different combinations of experimental datasets including the data at the lowest temperature also leads to unsatisfactory modeling results.
References
B. Rheingans and E.J. Mittemeijer: CALPHAD, 2015, DOI:10.1016/j.calphad.2015.04.013
R. Kampmann and R. Wagner: in Decomposition of Alloys: the Early Stages, P. Haasen, V. Gerold, R. Wagner, and M.F. Ashby, eds., Pergamon Press, Oxford, 1984, pp. 91–103
M. Volmer, A. Weber, Z. Phys. Chem. 119, 277–301 (1926)
R. Becker, W. Döring, Ann. Phys. 24, 719–752 (1935)
C. Zener, J. Appl. Phys. 20, 950–953 (1949)
H.B. Aaron, D. Fainstein, G.R. Kotler, J. Appl. Phys. 41, 4404–4410 (1970)
J.D. Robson, Acta Mater. 52, 4669–4676 (2004)
Z. Liu, V. Mohles, O. Engler, G. Gottstein, Comput. Mater. Sci. 81, 410–417 (2014)
J.W. Gibbs, The Collected Works of J (Green and Co., Williard Gibbs, Longmans, 1906)
V.A. Phillips, J.D. Livingston, Philos. Mag. 7, 969–980 (1962)
M. Takeda, N. Suzuki, G. Shinohara, T. Endo, J. van Landuyt, Phys. Status Solidi A 168, 27–35 (1998)
I.S. Servi, D. Turnbull, Acta Metall. Mater. 14, 161–169 (1966)
F.K. Legoues, H.I. Aaronson, Acta Metall. 32, 1855–1864 (1984)
M. Breu, W. Gust, B. Predel, Z. Metallkd. 82, 279–288 (1991)
W. Wagner, Acta Metall. Mater. 38, 2711–2719 (1990)
T. Ebel, R. Kampmann, R. Wagner, J. Phys. IV 3, 295–298 (1993)
G. Goerigk, H.G. Haubold, W. Schilling, J. Appl. Crystallogr. 30, 1041–1047 (1997)
X.D. Jiang, W. Wagner, H. Wollenberger, Z. Metallkd. 82, 192–197 (1991)
R. Hattenhauer, F. Haider, Scr. Metall. Mater. 25, 1173–1178 (1991)
R.P. Setna, J.M. Hyde, A. Cerezo, G.D.W. Smith, M.F. Chisholm, Appl. Surf. Sci. 67, 368–379 (1993)
R.P. Setna, A. Cerezo, J.M. Hyde, G.D.W. Smith, Appl. Surf. Sci. 76, 203–212 (1994)
A. Heinrich, T. Al-Kassab, R. Kirchheim, Surf. Interface Anal. 39, 240–245 (2007)
F.K. Legoues, H.I. Aaronson, Y.W. Lee, Acta Metall. 32, 1845–1853 (1984)
F.K. Legoues, Y.W. Lee, H.I. Aaronson, Acta Metall. 32, 1837–1843 (1984)
H.I. Aaronson, F.K. Legoues, Metall. Trans. A 23, 1915–1945 (1992)
R. Hattenhauer, P. Haasen, Philos. Mag. A 68, 1195–1213 (1993)
M.J. Stowell, Mater. Sci. Technol. 18, 139–144 (2002)
J.D. Robson, Mater. Sci. Technol. 20, 441–448 (2004)
J.D. Robson, M.J. Stowell, Philos. Mag. 84, 3101–3115 (2004)
J. Feder, K.C. Russell, J. Lothe, G.M. Pound, Adv. Phys. 15, 111–178 (1966)
M. Hillert: in Lectures on the Theory of Phase Transformations (1975), H.I. Aaronson, ed., 2nd ed., The Minerals, Metals and Materials Society, Warrendale, 1999, pp. 1–33
Q. Du, W.J. Poole, M.A. Wells, Acta Mater. 60, 3830–3839 (2012)
M. Perez, M. Dumont, D. Acevedo-Reyes, Acta Mater. 56, 2119–2132 (2008)
M. Palumbo, S. Curiotto, L. Battezzati, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 30, 171–178 (2006)
R. Wagner, R. Kampmann, and P.W. Voorhees: in Phase Transformations in Materials, G. Kostorz, ed., Wiley, Berlin, 2001, pp. 309–408
J.D. Eshelby, Solid State Phys. 3, 79–144 (1956)
R. Egerton: Electron Energy-Loss Spectroscopy in the Electron Microscope, Plenum Press, 1996
T. Malis, S.C. Cheng, R.F. Egerton, J. Electron. Micr. Tech. 8, 193–200 (1988)
S. Matsumura, M. Toyohara, Y. Tomokiyo, Philos. Mag. A 62, 653–670 (1990)
R. Döhl, M.P. Macht, V. Naundorf, Phys. Status Solidi A 86, 603–612 (1984)
Y.W. Lee, K.C. Russell, H.I. Aaronson, Scr. Metall. 15, 723–726 (1981)
G.J. Shiflet, Y.W. Lee, H.I. Aaronson, K.C. Russell, Scr. Metall. 15, 719–722 (1981)
E.J. Mittemeijer, Fundamentals of Materials Science (Springer Verlag, Heidelberg, 2010)
F. Liu, F. Sommer, C. Bos, E.J. Mittemeijer, Int. Mater. Rev. 52, 193–212 (2007)
B. Rheingans, Y. Ma, F. Liu, E.J. Mittemeijer, J. Non-Cryst. Solids 362, 222–230 (2013)
Y. Ma, B. Rheingans, F. Liu, E.J. Mittemeijer, J. Mater. Sci. 48, 5596–5606 (2013)
B. Rheingans, E.J. Mittemeijer, JOM 65, 1145–1154 (2013)
J.D. Robson, M.J. Jones, P.B. Prangnell, Acta Mater. 51, 1453–1468 (2003)
A. Heinrich: Ph.D. Thesis, Georg-August-Universität zu Göttingen, 2005.
J. W. Christian: The Theory of Transformations in Metals and Alloys, Pergamon Press, 2002
X.G. Lu, M. Selleby, B. Sundman, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 29, 68–89 (2005)
Y.A. Chang, L. Himmel, J. Appl. Phys. 37, 3567–3572 (1966)
B. Strauss, F. Frey, W. Petry, J. Trampenau, K. Nicolaus, S. Shapiro, J. Bossy, Phys. Rev. B 54, 6035–6038 (1996)
Acknowledgments
The Stuttgart Center for Electron Microscopy, Max Planck Institute for Intelligent Systems, Stuttgart, is gratefully acknowledged for access to TEM facilities. Dr. T. Bublat, formerly Max Planck Institute for Intelligent Systems, Stuttgart, is acknowledged for performing magnetisation measurements. One of the authors, BR, would like to thank Dr. E. A. Jägle, Max Planck Institute for Iron Research, Düsseldorf, for many fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted January 14, 2015.
Rights and permissions
About this article
Cite this article
Rheingans, B., Mittemeijer, E.J. Analysis of Precipitation Kinetics on the Basis of Particle-Size Distributions. Metall Mater Trans A 46, 3423–3439 (2015). https://doi.org/10.1007/s11661-015-2937-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-2937-x