Abstract
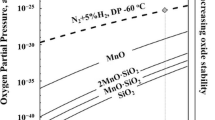
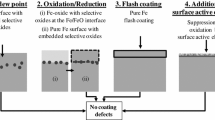
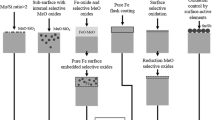
The selective oxidation of a CMnSi transformation-induced plasticity (TRIP) steel during intercritical annealing (IA) in a N2 + 10 pct H2 gas atmosphere with a dew point (DP) in the range from 213 K to 278 K (−60 °C to +5 °C) was investigated by transmission electron microscopy. The decarburization during IA resulted in a fully ferritic matrix at the TRIP steel surface. Annealing in high DP gas atmospheres resulted in a reduction of the oxide layer thickness at the surface and an increase of the depth of the subsurface internal oxidation. The experimental results were compared to the calculations of the DP for the transition from internal to external oxidation based on the Wagner model. The evolution of the surface oxide composition during annealing was analyzed thermodynamically by means of the chemical potential diagram for the surface oxides. In the high DP atmosphere conditions, mainly, Mn-rich xMnO·SiO2 (1 < x < 2) oxides were formed at the surface, while Si-rich xMnO·SiO2 (x < 1) oxides were formed by internal oxidation. The use of a high DP gas atmosphere is therefore advantageous to induce internal selective oxidation and reduce the amount of surface oxides. It also leads to the formation of Mn-rich xMnO·SiO2 (1 < x < 2) oxides.













Similar content being viewed by others
References
J. Mahieu, S. Claessens, B.C. De Cooman: Metall. Mater. Trans. A, 2001, vol. 32A, pp. 2905–08.
X.V. Eynde, J.P. Servais, M. Lamberigts: Surf. Interface Anal., 2003, vol. 35, pp. 1004–14.
J. Mahieu, S. Claessens, B.C. De Cooman, and F. Goodwin: The 6th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH 2004), Chicago, Illinois, 2004, Association for Iron and Steel Technology, pp. 529–38.
Y.F. Gong, H.S. Kim, B.C. De Cooman: ISIJ Int., 2008, vol. 48, pp. 1745–51.
Y.F. Gong, H.S. Kim, B.C. De Cooman: ISIJ Int., 2009, vol. 49, pp. 557–63.
L. Cho, M.S. Kim, Y.H. Kim, S.J. Lee, and B.C. De Cooman: Proceedings of the 8th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH 2011), Genova, Italy, 2011, Associazione Italiana di Metallurgia, pp. 145–52.
X.S. Li, S.I. Baek, C.S. Oh, S.J. Kim, Y.W. Kim: Scripta Mater., 2007, vol. 57, pp. 113–16.
A.R. Marder: Prog. Mater. Sci., 2000, vol. 45, pp. 191–271.
B. Mintz: Int. Mater. Rev., 2001, vol. 46, pp. 169–97.
L. Chen, H.S. Kim, S.K. Kim, B.C. De Cooman: ISIJ Int., 2007, vol. 47, pp. 1804–12.
C.E. Jordan, K.M. Goggins, A.R. Marder: Metall. Mater. Trans. A, 1994, vol. 25A, pp. 2101–09.
I. Hertveldt, B.C. De Cooman, S. Claessens: Metall. Mater. Trans. A, 2000, vol. 31A, pp. 1225–32.
S. Feliu, M. Pérez-Revenga: Acta Mater., 2005, vol. 53, pp. 2857–66.
M. Blumenau, M. Norden, F. Friedel, K. Peters: Steel Res. Int., 2010, vol. 81, pp. 1125–36.
C. Wagner: Z. für Elektrochem. Ber. Bunsenges. Phys. Chem., 1959, vol. 63, pp. 772–82.
G. Böhm, M. Kahlweit: Acta Metall., 1964, vol. 12, pp. 641–48.
R.A. Rapp: Corrosion, 1965, vol. 21, pp. 382–401.
Y. Niu, F. Gesmundo: Oxid. Met., 2006, vol. 65, pp. 329–55.
D. Huin, P. Flauder, J.B. Leblond: Oxid. Met., 2005, vol. 64, pp. 131–67.
J.B. Brunac, D. Huin, J.B. Leblond: Oxid. Met., 2010, vol. 73, pp. 565–89.
R. Rapp: Acta Metall., 1961, vol. 9, pp. 730–41.
L. Cho, S. Lee, M. Kim, Y. Kim, B.C. De Cooman: Metall. Mater. Trans. A, 2013, vol. 44A, pp. 362–71.
J. Takada, M. Adachi: J. Mater. Sci., 1986, vol. 21, pp. 2133–37.
H. Oikawa: Technology Reports, Tohoku University, 1983, vol. 48, pp. 7–77.
D.R. Lide: CRC Handbook of Chemistry and Physics, 86th ed., CRC Press, Boca Raton, 2005.
L. Cho, M.S. Kim, Y.H. Kim, B.C. De Cooman: Metall. Mater. Trans. A, 2013, vol. 44A, pp. 5081–95.
H. Liu, Y. He, L. Li: Appl. Surf. Sci., 2009, vol. 256, pp. 1399–1403.
H. Liu, Y. He, S. Swaminathan, M. Rohwerder, L. Li: Surf. Coat. Technol., 2011, vol. 206, pp. 1237–43.
Y. Suzuki, T. Yamashita, Y. Sugimoto, S. Fujita, S. Yamaguchi: ISIJ Int., 2009, vol. 49, pp. 564–73.
N. Birks, G.H. Meier, and F.S. Pettit: Introduction to the High Temperature Oxidation of Metals, 2nd ed., Cambridge University Press, New York, 2006, pp. 114–15.
J.M. Mataigne, M. Lamberigts, and V. Leroy: Developments in the Annealing of Sheet Steels, The Minerals, Metals and Materials Society (TMS), Warrendale, PA, 1992, pp. 511–28.
F. Wang: Oxid. Met., 1997, vol. 48, pp. 215–24.
Z. Liu, W. Gao, K.L. Dahm, F. Wang: Acta Mater., 1998, vol. 46, pp. 1691–700.
S. Guan, W. Smeltzer: Oxid. Met., 1994, vol. 42, pp. 375–91.
J. Töpfer, R. Dieckmann: Solid State Ionics, 2010, vol. 181, pp. 479–88.
I. Burn, S. Neirman: J. Mater. Sci., 1982, vol. 17, pp. 3510–24.
Acknowledgments
The authors gratefully acknowledge the support of Dr. Myung Soo Kim and Dr. Young Ha Kim of the POSCO technical Research Laboratories, Gwangyang, South Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 3, 2013.
Appendix: Application of Huin’s Model for Numerical Simulation
Appendix: Application of Huin’s Model for Numerical Simulation
Huin’s model[19] was used to calculate the internal oxidation of a ternary Mn-Si-Fe alloy where Fe is regarded as a matrix. During an isothermal annealing, Fe remains inert, whereas Mn and Si form MnO and SiO2, respectively. Numerical simulation was carried out by solving the following diffusion equations for Mn, Si, and oxygen.
Here, F Mn, F Si, and F O are the total mass fractions of Mn, Si, and oxygen (in all its possible forms, that is dissolved in the matrix or bound to precipitates), in ppm, respectively. C Mn, C Si, and C O are the dissolved mass fractions of Mn, Si, and oxygen, in ppm, respectively.
Several conditions should be considered for the calculation. First, counting atoms of element in all their possible forms (dissolved in the matrix or bound to precipitates) yields the following mass balance equations.
P MnO and \( P_{{{\text{SiO}}_{ 2} }} \) are the mass fractions of the precipitates of MnO and SiO2, in ppm, respectively. M Mn, M Si, and M O are the atomic mass of Mn, Si and oxygen, respectively. M MnO and \( M_{{{\text{SiO}}_{ 2} }} \) are the molecular mass of MnO and SiO2, respectively.
The expression for the local thermodynamic equilibrium governed by the laws of mass action yields the following equations:
Here, K MnO is the solubility product of MnO, in (ppm)2. \( K_{{{\text{SiO}}_{ 2} }} \) is the solubility product of SiO2, in (ppm)3. Equation [8] indicates that there will be no precipitation when the product of C Mn and C O is less than the value of K MnO. When the product of C Mn and C O exceeds the value of K MnO, C Mn and C O will react until the product of C Mn and C O equals to the value of K MnO. A similar relation holds in the case of SiO2.
The one-dimension problem is considered, with the spatial coordinate, the depth below the free surface, being denoted x. All quantities at time t (denoted with an upper index(0)) being assumed to be known, the problem is to determine all quantities at time t + ∆t (denoted without any index). The spatial discretization involving N nodes x 1 = 0, x 2, …, x N is introduced (Figure 14). In practice, node 1 will represent the outer surface of the sheet in contact with the annealing atmosphere, and node N will be located far below the surface.
Spatial discretization[19]
Discretization of the ordinary differential equations (Eq. [6]) was done by finite difference method using an explicit Euler scheme order 1, as given in Eq. [10].
Here, the time-step, ∆t, is taken as 1/10 of the quantities (∆x k )2/D O for the sake of numerical accuracy. These equations do not apply at the boundary nodes x 1 and x N . At node x 1, Eq. [11] is adopted instead of (10) and the theoretical basis on the treatment of the boundary condition is described in the Huin’s original work[19,20]:
At node x 1, if it is assumed that chemical equilibrium is rapidly achieved, the dissolved oxygen at the surface, \( C_{\text{O}}^{{ ( {\text{S)}}}} \), is obtained by combining Eqs. [2], [3], and [4]:
At node x N , deep in the steel sheet, it is assumed that the Mn and Si concentrations remain constant at their nominal value given by the steel composition, and that no MnO and SiO2 are present:
Here, \( C_{\text{Mn}}^{{ ( {\text{O)}}}} \) and \( C_{\text{Si}}^{{ ( {\text{O)}}}} \) are the Mn and Si mass concentrations in the steel.
Finally, if all the values of F Mn(x k ), F Si(x k ), F O(x k ), C Mn(x k ), C Si(x k ), C O(x k ), P MnO(x k ), and \( P_{{{\text{SiO}}_{ 2} }} \)(x k ) at every node are known at the time t, the calculation of the values at t + ∆t can be carried out by following sequences:
-
1.
At node x 1, F Mn(x 1) and F Si(x 1) are obtained by Eq. [11]. C Mn(x 1) and C Si(x 1) then follow by solving the mass balance equations (Eq. [7]). P MnO(x 1 ) and \( P_{{{\text{SiO}}_{ 2} }} \)(x 1) are calculated by thermodynamic equilibrium (Eqs. [8] and [9]) as the term C O(x 1) in Eqs. [8] and [9] is already known by Eq. [12]. F O(x 1) is finally obtained by Eq. [7].
-
2.
At nodes x 2, x 3, …, x N , the precipitation of SiO2, which has a low solubility product, is considered prior to that of MnO. F Si(x k ) is obtained from the finite difference method (Eq. [10]). If SiO2 precipitate, none-zero \( P_{{{\text{SiO}}_{ 2} }} \) can be obtained by the calculation of thermodynamic equilibrium. Because the C Si(x k ) and C O(x k ) terms in Eq. [9] are unknown, P MnO should be obtained by expressing C Mn(x k ) and C O(x k ) in terms of F Mn(x k ) and F O(x k ) using Eq. [7]. This yields Eq. [14].
$$ \left( {F_{\text{Si}} - \frac{{M_{\text{Si}} }}{{M_{{{\text{SiO}}_{ 2} }} }}P_{{{\text{SiO}}_{ 2} }} } \right) \times \left( {F_{\text{O}} - \frac{{M_{\text{O}} }}{{M_{\text{MnO}} }}P_{\text{MnO}} - 2\times \frac{{M_{\text{O}} }}{{M_{{{\text{SiO}}_{ 2} }} }}P_{{{\text{SiO}}_{ 2} }} } \right)^{ 2} { = }K_{{{\text{SiO}}_{ 2} }} . { } $$(14)Equation [14] can be solved numerically using Newton–Raphson method. C Si(x k ) and C O(x k ). This is followed by solving Eq. [7]. In the same manner, F Mn(x k ) is obtained from Eq. [10]. Non-zero values of P MnO can be obtained by Eqs. [7] and [8], which yields Eq. [15].
$$ \left( {F_{\text{Mn}} - \frac{{M_{\text{Mn}} }}{{M_{\text{MnO}} }}P_{\text{MnO}} } \right) \times \left( {F_{\text{O}} - \frac{{M_{\text{O}} }}{{M_{\text{MnO}} }}P_{\text{MnO}} - 2\times \frac{{M_{\text{O}} }}{{M_{{{\text{SiO}}_{ 2} }} }}P_{{{\text{SiO}}_{ 2} }} } \right) = K_{\text{MnO}} . { } $$(15)Equation [15] is solved numerically using Newton–Raphson method. C Mn(x k ) and C O(x k ) are then updated by the mass balance equations.
-
3.
At node x N , all the parameter values are kept constant, as expressed in Eq. [13].
Rights and permissions
About this article
Cite this article
Cho, L., Jung, G.S. & De Cooman, B.C. On the Transition of Internal to External Selective Oxidation on CMnSi TRIP Steel. Metall Mater Trans A 45, 5158–5172 (2014). https://doi.org/10.1007/s11661-014-2442-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-014-2442-7