Abstract
Summary
This study assessed the cost-effectiveness of continued denosumab treatment, compared with discontinuation of denosumab after one dose, for the treatment of postmenopausal osteoporosis in Taiwan, using real-world fracture reduction effectiveness and cost data. Outcomes indicate that continued denosumab treatment produces an incremental cost-effectiveness ratio of USD $16,743 per QALY.
Purpose
To evaluate the cost-effectiveness of continued denosumab use versus discontinuation after one dose, for the treatment of postmenopausal osteoporosis in Taiwan, using real-world fracture reduction effectiveness and cost data.
Methods
A Markov cohort model was used to evaluate the lifetime costs and QALYs associated with continued denosumab treatment versus discontinuation of treatment after one dose. The evaluation was conducted from the perspective of Taiwan’s healthcare system and used a discount rate of 3% per annum. The patient population consisted of postmenopausal women with osteoporosis with a mean age of 77 years who initiated denosumab treatment. Fracture reduction effectiveness data, baseline fracture rates, mortality data, and costs of fracture were informed by Taiwan’s National Health Insurance Research Database.
Results
Model outcomes showed that continued treatment with denosumab produced an expected gain of 0.042 QALYs and an incremental cost of USD $704, compared with discontinuation of denosumab after one dose. This corresponds to an incremental cost-effectiveness ratio of USD $16,743 per QALY gained. Probabilistic and scenario analysis showed that results are stable to variations in model assumptions and parameters.
Conclusion
In a real-world setting, at a cost per QALY threshold equivalent to gross domestic product per capita in 2020 in Taiwan (USD $30,038), continued treatment with denosumab in postmenopausal women with osteoporosis is cost-effective compared with treatment discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common condition, whose prevalence is increasing with the progressively aging population in Asia. Fragility fractures are the main consequence of osteoporosis, which, as well as resulting in increased mortality and diminished health-related quality of life (HRQoL), also produce a substantial economic burden [1].
Denosumab is a fully human monoclonal antibody that targets and inhibits RANK-L, a ligand which stimulates bone resorption, with high specificity. Denosumab is currently approved as a treatment for postmenopausal osteoporosis in more than 80 healthcare markets globally [2,3,4,5], including in Taiwan, where it has been marketed since 2011 [6], and is one of the most frequently used antiresorptive treatments under Taiwan’s National Health Insurance system [7]. As with most pharmacological treatments, compliance with osteoporosis therapies has been shown to be an important factor in achieving treatment success [8].
During the past decade, several economic models have been published evaluating the cost-effectiveness of denosumab compared with either no treatment or other pharmacological therapies [9,10,11,12]. All these studies used efficacy data from randomized clinical trials (RCT), or network meta-analyses of RCTs, to inform the fracture reduction effects of pharmacological therapy, thereby making the assumption that the treatment efficacy observed in trials translates to clinical practice. To address this potential limitation, the cost-effectiveness of denosumab was assessed using treatment effectiveness data from a robust real-world study.
A retrospective database study evaluating the effectiveness and safety of denosumab in clinical practice among women with postmenopausal osteoporosis in Taiwan and Hong Kong using National Health Insurance claims data was completed in 2019 [13], referred to the Taiwan Real-World Study (TWRWS) hereinafter. Employing the propensity score (PS) matching approach, this study compared the real-world clinical fracture incidence in patients who received at least 2 doses of denosumab (referred to as the “treatment cohort”) and patients who discontinued after 1 dose of denosumab (referred to as “off-treatment cohort”) between 2012 and 2016, using an index date of 225 days after the initial dose. The off-treatment cohort was selected to minimize confounding relating to the initial treatment decision, and because this patient group can be considered a proxy for a “no treatment” cohort, given that any fracture reduction benefit from the initial denosumab dose is unlikely to persist beyond the study index date. PS matching was undertaken based on 59 baseline variables, all of which were balanced between cohorts following the matching process, defined as exhibiting a standardized mean difference < 0.1. Results of the study showed that the risk of hip fracture, clinical vertebral fracture, and non-vertebral fracture was reduced in the Taiwanese treatment cohort by 38% (hazard ratio, 0.62 [95% CI: 0.52, 0.75]), 37% (hazard ratio, 0.63 [95% CI: 0.52, 0.75]), and 38% (hazard ratio, 0.62 [95% CI: 0.53, 0.73), respectively.
The objective of this economic analysis is to evaluate the cost-effectiveness of continued treatment with denosumab versus discontinuation of denosumab after one dose in postmenopausal women with osteoporosis in Taiwan, using fracture reduction effectiveness outcomes from the TWRWS. The study design was selected to align with the design of the TWRWS as closely as possible, and therefore assessed the cost-effectiveness of persistence with denosumab versus non-persistence, rather than the cost-effectiveness of treatment versus no treatment per se. However, given that patients in the “off-treatment cohort” received only a single dose of denosumab, outcomes are also likely to represent a reasonable proxy for the latter comparison. To the best of the authors’ knowledge, this study is the first to use denosumab treatment effectiveness data from a real-world study to inform an economic analysis.
Methods
Model overview
A cost-effectiveness model, whose structure has been described previously [9, 10, 14], was adapted to evaluate lifetime costs and quality-adjusted life years (QALYs) associated with continued denosumab use (treatment cohort) and discontinuation of denosumab after one dose (off-treatment cohort). The analysis was conducted from the perspective of Taiwan’s healthcare system and used an annual discount rate of 3% for costs and health outcomes, in line with local guidelines [15]. Costs were assessed in 2020 US dollars.
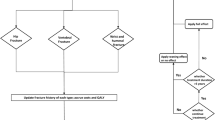
The model used a Markov cohort structure (shown in Fig. 1) to assess the incidence of hip, vertebral, and “other” (wrist or distal forearm) fractures over time. All patients start in the “well” state, and in each 6-month model cycle are at risk of experiencing a fragility fracture (hip, vertebral, or “other” fracture) or dying. To track the long-term impact of fractures on health-related quality of life (HRQoL), healthcare costs, and mortality, after 1 year in the hip fracture or vertebral fracture state, patients transition to the post-hip fracture and post-vertebral fracture states, respectively. After 1 year in the “other” fracture state, patients transition back to the well state.
The model uses a hierarchical structure, based on the severity of fracture types, with hip fracture being the most severe, and “other” fracture the least severe. Patients in hip fracture states cannot sustain a subsequent vertebral or “other” fracture, and patients in the vertebral fracture states cannot sustain an “other” fracture. To adjust for the underestimation of vertebral and “other” fracture incidence imposed by this structure, a correction factor was implemented, where the incidence of overlooked fractures and their impact on costs, HRQoL, and mortality was estimated independently of the Markov process, as described previously [14].
Patient population
The modelled population was based on participants in the TWRWS [13]: postmenopausal women with osteoporosis who initiated treatment with denosumab, with a mean age of 77 years. The model simulated patients from the time at which the first dose of denosumab was administered.
Treatment duration
Patients in the off-treatment cohort received a single dose of denosumab, covering a 6-month period. Patients in the treatment cohort were assumed to receive denosumab treatment for an intended duration of 5 years, consistent with previous economic analyses [9, 10, 14].
Following the second dose of denosumab in the treatment cohort, treatment persistence was modelled using a previously published modelling framework [16]. In each cycle, a proportion of patients discontinued treatment, based on a discontinuation rate of 0.317 per patient-year among patients in the treatment cohort of the TWRWS, which was converted to a discontinuation probability of 14.7% per 6-month model cycle, assuming a constant rate over time.
Clinical evidence has shown that the fracture reduction benefit of osteoporosis treatment continues for some time after discontinuation, rather than stopping immediately [16]. Therefore, in line with previous peer-reviewed economic analyses of denosumab, the assumption was made that treatment benefit decreases linearly to 0 over a period of 2 years following discontinuation [10, 17].
Clinical inputs
Fracture incidence in untreated patients was informed by annual rates of hip, vertebral, and “other” (wrist or distal forearm) fracture from the off-treatment cohort of the TWRWS [13] (non-PS matched). Fractures were identified using diagnosis codes from the International Classification of Diseases, 9th Revision, Clinical Modification until December 2015, and the International Classification of Diseases, 10th Revision, Clinical Modification thereafter [18], with validation of hip fractures conducted by external medical chart review. Fractures occurring on the same date as a motor vehicle accident were excluded, to increase the probability that fractures were due to osteoporosis.
To inform fracture rates in denosumab-treated patients, hazard ratios of fracture (PS matched) for the treatment versus off-treatment cohort of the TWRWS were applied to fracture rates in untreated patients. As with previous analyses, a hazard ratio for nonvertebral fracture was used to inform “other” fracture incidence [9, 10]. Hazard ratios were applied for persistent patients in the treatment cohort during the 5-year treatment course (and during the offset period after treatment discontinuation), and for the first cycle of the model for the off-treatment cohort, to account for the single dose of denosumab received by these patients. Fracture rates and treatment effectiveness data used in the model are shown in Table 1.
Mortality
Probabilities of death in the model were derived from Chang et al. (2016) [19]: a study comparing mortality and healthcare costs among postmenopausal women following osteoporotic fracture with age- and comorbidity-matched controls, using data from Taiwan’s National Health Insurance Research Database (NHIRD). Age-specific mortality for patients in the “well” state was informed by death rates in matched control groups, estimated as a weighted average across control groups for all fracture types. To account for the increased mortality risk due to fracture, age-specific relative risks of death for the case versus control cohort were applied in the first year after hip, vertebral, and “other” fracture, and for the second and following years after hip and vertebral fracture. Since the study did not explicitly report relative risks of death for “other” fractures, these values were estimated as a weighted average of mortality outcomes for wrist and upper end humerus fracture. Mortality inputs used in the model are shown in Table 1.
Consistent with previous denosumab economic analyses [9, 10], the model assumed that excess fracture-related mortality persists for 8 years after the event for hip and vertebral fracture, and 1 year after the event for “other” fracture.
Costs
The model included two categories of cost: medical costs due to fracture and treatment costs. Costs were adjusted to 2020 values using inflation data for Taiwan [20], and converted from New Taiwan dollars to US dollars where required, using an exchange rate of 0.0334 USD per TWD [21].
Age-specific medical costs due to fracture were informed by Taiwan’s National Health Insurance Research Database (NHIRD) [19], and included a first-year cost for all fracture types (hip, vertebral, and “other”), and a recurring annual cost in the second and subsequent years after the event for hip and vertebral fracture. As with fracture-related mortality inputs, the first-year cost of “other” fractures was informed by a weighted average of the cost of wrist and upper end humerus fracture.
Treatment costs included the annual drug cost of denosumab (Prolia®), informed by Taiwan’s National Health Insurance Administration [22], and an annual treatment management cost, which consisted of a routine healthcare visit every year, a bone densitometry (DXA) scan every 2 years, and a nurse visit every 6 months for denosumab administration [23]. Cost inputs used in the model are shown in Table 1.
Health-related quality of life
Age-specific general population EQ-5D scores for Chinese women [24] were used to inform health-related quality of life (HRQoL) for patients in the “well” state. To account for the HRQoL reduction due to fracture, utility multipliers were applied to “well” state utilities for the first year after hip, vertebral, and “other” fracture, and in the second and following years after hip and vertebral fracture. These values were taken from an analysis of data from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) [25]. Inputs relating to HRQoL are shown in Table 1.
Analysis
The model assessed outcomes in terms of total discounted lifetime costs and QALYs for each intervention. In the base case, results were produced using point estimates for each model input. The economic analysis used the annual nominal gross domestic product (GDP) per capita in Taiwan (USD $30,038 [26]) as a willingness-to-pay threshold per QALY gained: a conservative approach based on the commonly used range of one to three times national GDP per capita in countries without an explicit threshold [27].
Uncertainty in model outcomes was addressed through scenario analyses, which explored the impact of (1) assuming full treatment persistence in the treatment cohort and (2) adjusting fracture rates to account for the ageing of the cohort over time. This second scenario analysis was conducted because fracture rates typically increase as patients age. However, age-stratified fracture rates were not available from the Taiwan real-world study, so were assumed to remain constant over time in the base case. To explore this assumption, as a scenario analysis, external data on the relative incidence of fractures in Swedish women of different ages [28] were used to estimate changing fracture rates over time for the Taiwanese cohort.
Uncertainty was also assessed via probabilistic sensitivity analysis, where model parameters were simultaneously stochastically varied, according to probability distributions informed by their point estimates and standard errors (taken from publications where available or assumed to be 10% of the mean otherwise), for 1,000 model iterations. This allowed the probability that each intervention is cost-effective to be quantified over a range of willingness to pay thresholds.
Results
Base case cost-effectiveness results (displayed in Table 2) show that continued treatment with denosumab for up to 5 years (the treatment cohort) produces a reduction in fractures compared with discontinuation of denosumab after one dose (the off-treatment cohort); a total of 45 fractures are avoided per 1,000 patients over a 10-year period. This fracture reduction translates into a lifetime gain of 0.023 life years and 0.042 QALYs per patient. Continued treatment with denosumab is associated with a higher drug cost and treatment management cost (increase of $994), which is partially offset by a $290 reduction in lifetime fracture costs, producing a total incremental cost of $704. Continued denosumab treatment therefore produces an incremental cost-effectiveness ratio (ICER) of $16,743 per QALY compared with discontinuation of denosumab after one dose.
Scenario analysis results (displayed in Table 3) show that assuming 100% persistence among patients who continue denosumab treatment increases the ICER versus patients who discontinue after one dose to $20,882 per QALY. Contrastingly, adjusting fracture rates to account for increasing fracture risk as the cohort ages reduces the ICER to $14,452.
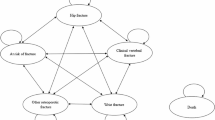
Probabilistic sensitivity analysis results are displayed as cost-effectiveness acceptability curves in Fig. 2. These results show that continued treatment with denosumab is cost-effective in 99.0% of stochastic iterations at a cost per QALY threshold equivalent to Taiwan’s GDP per capita (USD $30,038 [26]). Continued denosumab treatment produces the highest number of QALYs in all stochastic iterations, reflected by the fact that this strategy becomes cost-effective in 100% of samples as the willingness to pay threshold rises.
Cost-effectiveness acceptability curves—showing the number of iterations in which continued denosumab treatment (treatment) and discontinuation of denosumab after one dose (off-treatment) are cost-effective over a range of willingness to pay thresholds. QALY, quality-adjusted life year; USD, United States dollar; WTP, willingness to pay
Discussion
This study assessed the cost-effectiveness of continued treatment with denosumab (the “treatment cohort”) with discontinuation of denosumab after one dose (the “off-treatment cohort”), using real-world effectiveness and cost data from Taiwan’s NHIRD. Results show that, compared with discontinuation after one dose, continued treatment with denosumab produces a gain of 0.042 QALYs, due to the fracture reduction benefit of treatment, and an incremental cost of USD $704 (despite a reduction in fracture costs), due to the higher drug cost. Continued denosumab treatment therefore produces an ICER of $16,743 per QALY, indicating that this strategy is cost-effective at a cost per QALY threshold equivalent to Taiwan’s GDP per capita (USD $30,038 [26]). As suggested by the World Health Organization [27], in settings without an explicit willingness-to-pay threshold, interventions with an ICER below three times local GDP per capita are commonly considered cost-effective, and those with an ICER below one times GDP per capita considered highly cost-effective. Scenario analysis and probabilistic sensitivity analysis show that cost-effectiveness results are generally stable to variation in model assumptions and parameters.
The modelling approach used in this study is generally consistent with previous work in the field. The model structure has been used to evaluate the cost-effectiveness of denosumab in various settings, including Sweden [9, 29], the USA [10], Canada [30], and Thailand [17]. Like a number of prior economic evaluations of osteoporosis therapies [31,32,33], this structure is based on the widely accepted International Osteoporosis Foundation reference model.
To the best of the authors’ knowledge, this study is distinct from previous economic evaluations of denosumab in two ways. First, model inputs were taken from a single real-world source (the NHIRD) wherever possible. In the current study, real-world effectiveness data were used to inform relative fracture incidence between arms. By contrast, previous economic analyses [9, 10, 17, 29, 30] used treatment efficacy data from RCTs, or network meta-analyses of RCTs, which predominantly relied on the FREEDOM trial [2] (the pivotal phase III denosumab RCT) for the fracture reduction benefit of denosumab. Furthermore, in the current study, fracture rates, treatment discontinuation data, fracture costs, and excess mortality were all informed by the NHIRD, whereas previous evaluations used data from a variety of sources. Using real-world data addresses issues with the generalizability of RCT evidence to clinical practice. For instance, real-world patients are likely to exhibit lower treatment compliance, and are likely to be more diverse in characteristics than participants in RCTs. Additionally, according to reimbursement guidance from Taiwan’s National Insurance Program, only patients with a prior fracture are eligible for osteoporosis treatment [34]. This is reflected in the substantially higher proportion of patients with a fracture at baseline in the TWRWS, compared with the FREEDOM trial [2].
A second way in which the current study differs from previous analyses is the choice of comparators. This comparison is consistent with the study design of the TWRWS, which assessed fracture outcomes in patients who received at least two doses of denosumab versus those who discontinued denosumab after one dose to minimize confounding; a real-world study comparing denosumab-treated patients with untreated patients would be subject to bias, since the two patient groups are likely to differ in terms of characteristics and risk factors. To align as closely with the available evidence as possible, the current evaluation modelled the two cohorts as they were defined in the TWRWS study, and therefore assessed the cost-effectiveness of persistence with denosumab, rather than using the “off-treatment” cohort to represent a “no treatment” arm. However, considering that the residual fracture reduction benefit from the initial dose of denosumab in the off-treatment TWRWS cohort is likely to be minimal, outcomes of this evaluation are also likely to represent a reasonable proxy for the real-world cost-effectiveness of denosumab versus no treatment.
As with all economic evaluations, this study has several limitations. First, while model parameters were sourced from NHIRD data wherever available, this was not possible in all cases. Of note, general population health-related quality of life (HRQoL) scores were informed by mainland Chinese data. Although the populations of mainland China and Taiwan are similar, healthcare systems, and potentially health state valuations, differ between the two locations. Therefore, further research is required to produce Taiwan-specific data to inform future economic analyses.
Second, the Taiwan real-world study did not include all types of fragility fracture; only hip, vertebral, humerus, and distal forearm fractures were considered. Although these are regarded as the most important fracture types (in terms of cost and impact on HRQoL and mortality), only including these locations means that the incidence of “other” fractures (i.e., non-hip, non-vertebral fractures) in the model is likely to be underestimated. This omission leads to an understatement of the burden of disease overall, and likely provides a conservative estimate of the cost-effectiveness of continued denosumab treatment; lower fracture rates lead to a smaller absolute fracture reduction for the treatment cohort, and therefore a smaller QALY gain and cost reduction from avoided fractures.
Third, this evaluation assumes that fracture rates in untreated patients remain constant over time, due to a lack of age-specific fracture data from the Taiwan real-world study. In reality, fracture incidence generally increases with age [28]. In a scenario analysis, the impact of this assumption was explored by adjusting fracture rates for age, using external data on age-specific fracture incidence in Swedish women [28]. Results of this scenario indicate that the assumption of constant fracture rates is likely to underestimate the cost-effectiveness of continued denosumab treatment.
Fourth, the index date of the Taiwan real-world study does not align exactly with the cycle length of the model. Study outcomes were recorded starting from 6 months and 45 days after the first dose of denosumab, whereas each cycle of the model begins at the time of denosumab administration. However, given the chronic nature of osteoporosis and the relatively long model cycle length, this discrepancy is unlikely to materially affect results.
Finally, as with any evaluation based on observational effectiveness data, it is impossible to rule out residual bias due to unobserved confounders. However, the design of the TWRWS (where both cohorts were selected based on treatment with denosumab, and propensity score matching was conducted using a substantial number of covariates) is likely to minimize the risk of confounding. Information on patients’ smoking and alcohol use, body mass index (BMI), and bone mineral density (BMD) were not available from Taiwan’s NHIRD, and therefore could not be included as covariates in the propensity score matching process. However, the prevalence of smoking and alcohol use is less than 5% among the elderly in Taiwan [35], and a subset analysis using records from the Chang Gung Research Database [36] showed that BMI and BMD were balanced between cohorts at baseline. Therefore, it is unlikely that these unobserved variables were associated with a substantial confounding effect.
Despite these limitations, this study provides clear evidence that continued treatment with denosumab is cost-effective compared with discontinuation after one dose, from the perspective of Taiwan’s National Health Insurance system, at a cost per QALY threshold equivalent to GPD per capita. Probabilistic sensitivity analysis and scenario analyses highlight that model conclusions are generally robust to uncertainty. Further research in this area is required, both in providing suitable Taiwan-specific data for future economic evaluations of osteoporosis treatments and in providing real-world evidence of denosumab’s effectiveness in other settings.
Conclusion
Results of this cost-effectiveness analysis indicate that continued treatment with denosumab for up to 5 years is highly cost-effective compared with discontinuation of denosumab after one dose in a real-world setting, from the perspective of Taiwan’s healthcare system, using a willingness-to-pay threshold equivalent to national GDP per capita.
Data availability
Not applicable.
Code availability
Not applicable.
References
Cheung EYN, Tan KCB, Cheung C-L, Kung AWC (2016) Osteoporosis in East Asia: Current issues in assessment and management. Osteoporosis and Sarcopenia 2(3):118–133
Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński DE, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. The Lancet Diabetes & Endocrinology 17(S2213):8587
Australian Government; Department of Health and Ageing (2011) Australian public assessment report for denosumab. Available from: https://www.tga.gov.au/sites/default/files/auspar-prolia.pdf. Accessed 11 Apr 2021
National Institute for Health and Clinical Excellence (2010) Final appraisal determination Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Available from: https://www.nice.org.uk/guidance/ta204/documents/osteoporotic-fractures-denosumab-final-appraisal-determination-document2. Accessed 11 Apr 2021
Food and Drug Administration; Ministry of Health and Welfare. Western medicine, medical equipment, cosmetics license inquiry. Available from: https://info.fda.gov.tw/MLMS/H0001D.aspx?Type=Lic&LicId=10000918. Accessed 25 Aug 2021
National Health Insurance Administration; Ministry of Health and Welfare. Analysis of drug usage. Available from: https://www.nhi.gov.tw/Content_List.aspx?n=5AA7CAFFF61CB16D&topn=5FE8C9FEAE863B46. Accessed 25 Aug 2021
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022
Jönsson B, Ström O, Eisman JA, Papaioannou A, Siris ESE, Tosteson A, Kanis JA (2011) Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22(3):967–982
Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D (2013) Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 11:485–497
Silverman S, Agodoa I, Kruse M, Parthan A, Orwoll E (2015) Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J Osteoporos 2015:627631
Raje N, Roodman GD, Willenbacher W, Shimizu K, García-Sanz R, Terpos E, Kennedy L, Sabatelli L, Intorcia M, Hechmati G (2018) A cost-effectiveness analysis of denosumab for the prevention of skeletal-related events in patients with multiple myeloma in the United States of America. J Med Econ 21(5):525–536
Lai E.C.-C., Lin T.-C., Lange J.L., Chen L., Wong I., Sing C.-W., Cheung C.-L., Shao S.-C., and Yang Y.-H.K., Effectiveness of denosumab for fracture prevention in real-world postmenopausal women with osteoporosis: retrospective cohort study. 2021, Amgen Inc.: Thousand Oaks, California, USA; OSIN-D-21–00989.
Svedbom A, Hadji P, Hernlund E, Thoren R, McCloskey E, Stad R, Stollenwerk B (2019) Cost-effectiveness of pharmacological fracture prevention for osteoporosis as prescribed in clinical practice in France, Germany, Italy, Spain, and the United Kingdom. Osteoporos Int 30(9):1745–1754
Taiwan Society for Pharmacoeconomics and Outcome Research (2014) Health technology assessment guidelines. Available from: http://www.taspor.org.tw/. Accessed 11 Apr 2021
Ström O, Borgström F, Kanis J, Jönsson B (2009) Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 20(1):23–34
Pongchaiyakul C, Nanagara R, Songpatanasilp T, Unnanuntana A (2020) Cost-effectiveness of denosumab for high-risk postmenopausal women with osteoporosis in Thailand. J Med Econ 23(7):776–785
World Health Organization (2021) International Statistical Classification of Diseases and Related Health Problems (ICD). Available from: https://www.who.int/standards/classifications/classification-of-diseases. Accessed 25 Aug 2021
Chang C-Y, Tang C-H, Chen K-C, Huang K-C, Huang K-C (2016) The mortality and direct medical costs of osteoporotic fractures among postmenopausal women in Taiwan. Osteoporos Int 27(2):665–676
Statista. Taiwan: Inflation rate from 1984 to 2021. Available from: https://www.statista.com/statistics/727598/inflation-rate-in-taiwan/. Accessed 3 June 2020
Xe. Currency Converter: USD to TWD. Available from: https://www.xe.com/currencyconverter/convert/?Amount=1&From=USD&To=TWD#:~:text=XE%20Currency%20Converter%3A%201%20USD%20to%20TWD%20%3D%2028.5290%20Taiwan%20New%20Dollars. Accessed 3 June 2020
National Health Insurance Administration; Ministry of Health and Welfare (2012) Health insurance drug item query; denosumab. Available from: https://www1.nhi.gov.tw/QueryN/Query1.aspx?n=FC660C5B07007373&sms=36A0BB334ECB4011&topn=3185A4DF68749BA9&upn=80567D1327F69CB9. Accessed 3 June 2020
National Health Insurance Administration; Ministry of Health and Welfare. Available from: https://www.nhi.gov.tw/query/query2.aspx. Accessed 11 Apr 2021
Sun S, Chen J, Johannesson M, Kind P, Xu L, Zhang Y, Burström K (2011) Population health status in China: EQ-5D results, by age, sex and socio-economic status, from the National Health Services Survey 2008. Qual Life Res 20(3):309–320
Svedbom A, Borgstöm F, Hernlund E, Ström O, Alekna V, Bianchi ML, Clark P, Curiel M, Dimai HP, Jürisson M (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures—results from the ICUROS. Osteoporos Int 29(3):557–566
Statistical Bureau; Republic of China (Taiwan) (2019) National Statistics/Statistical Tables. Available from: http://eng.stat.gov.tw/ct.asp?xItem=37408&CtNode=5347&mp=5. Accessed 1 Dec 2020
Marseille E, Larson B, Kazi.S., Kahn J.G., and Rosen S. (2015) Thresholds for the cost–effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization 93(118):124
Kanis J, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Parthan A, Kruse M, Agodoa I, Silverman S, Orwoll E (2014) Denosumab: a cost-effective alternative for older men with osteoporosis from a Swedish payer perspective. Bone 59:105–113
Chau D, Becker D, Coombes M, Ioannidis G, Adachi J, Goeree R (2012) Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in Canada. J Med Econ 15(sup1):3–14
Ström O, Borgström F, Sen S, Boonen S, Haentjens P, Johnell O, Kanis J (2007) Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries-an economic evaluation based on the fracture intervention trial. Osteoporos Int 18(8):1047–1061
Borgström F, Jönsson B, Ström O, Kanis J (2006) An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting. Osteoporos Int 17(12):1781–1793
Kanis JA, Adams J, Borgström F, Cooper C, Jönsson B, Preedy D, Selby P, Compston J (2008) The cost-effectiveness of alendronate in the management of osteoporosis. Bone 42(1):4–15
National Health Insurance Administration; Ministry of Health and Welfare. Drug payment regulations. Available from: https://www.nhi.gov.tw/Content_List.aspx?n=E70D4F1BD029DC37&topn=5FE8C9FEAE863B46. Accessed 11 Apr 2021
Health Promotion Administration; Ministry of Health and Welfare; Taiwan. Survey results of Chinese smoking behavior. Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=1718&pid=9913. Accessed 25 Aug 2021
Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, Lai CC, Lai ECC (2019) The Chang Gung Research Database—a multi‐institutional electronic medical records database for real‐world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf 28(5):593–600
Funding
This study was funded by Amgen Asia Holding Limited.
Author information
Authors and Affiliations
Contributions
All authors participated in designing the study. BJ, HL, and BS conducted the analysis and drafted the manuscript. ECCL and HO provided clinical expertise, input into the development of the modelling approach, and advised on local inputs and adapting the model to a Taiwanese setting. All authors reviewed and provided revisions to the manuscript and approved the final version for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
Edward Chia-Cheng Lai received grants from the Ministry of Science and Technology of Taiwan, the Drug Relief Foundation (TDRF) of Taiwan, Hong Kong Community Recovery Fund, Amgen, AbbVie, Taketa, Sanofi, and IQVIA Ltd. Huang-tz Ou received a grant from the Ministry of Science and Technology of Taiwan. Ben Johnson, Hong Li, and Björn Stollenwerk are employees of Amgen and own Amgen stock.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnson, B., Lai, E.CC., Ou, Ht. et al. Real-world cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis in Taiwan. Arch Osteoporos 16, 155 (2021). https://doi.org/10.1007/s11657-021-01020-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-01020-6