Abstract
Summary
Bone mass achievement predicts later fracture risk. This population-based study describes bone mineral density (BMD) levels and associated factors in Norwegian adolescents. Compared with international reference ranges, BMD levels appear higher and physical activity levels are positively associated with BMD.
Purpose
Norway has one of the highest reported incidences of osteoporotic fractures. Maximisation of peak bone mass may prevent later fractures. This population-based study compared BMD levels of Norwegian adolescents with international reference ranges and explored associated factors.
Methods
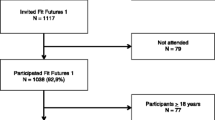
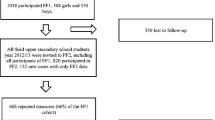
All first-year upper-secondary school students, aged 15–19 years in the Tromsø region were invited to the Fit Futures study in 2010–2011. Over 90 % of the invited participants attended, 508 girls and 530 boys. BMD was measured at total hip, femoral neck and total body by dual X-ray absorptiometry. Lifestyle variables were collected by self-administered questionnaires and interviews. All analyses were performed sex stratified, using linear regression models.
Results
In girls, mean BMD (SD) was 1.060 g/cm2 (0.124), 1.066 g/cm2 (0.123) and 1.142 g/cm2 (0.077) at the total hip, femoral neck and total body, respectively. In boys, corresponding values were 1.116 (0.147), 1.103 (0.150) and 1.182 (0.097), with significant higher values than the Lunar pediatric reference at 16 years of age. In girls, height and self-reported intensive physical activity of more than 4 h a week and early sexual maturation were positively associated with BMD at both femoral sites (p < 0.047). Among boys age, height, body mass index, physical activity and alcohol intake were positively (p < 0.038), whereas early stages of sexual maturation and smoking was negatively (p < 0.047) related to BMD.
Conclusions
Despite the heavy fracture burden, Norwegian adolescents’ BMD levels are higher than age-matched Caucasians. Physical activity is associated with 1 SD increased BMD levels in those involved in competition or hard training.


Similar content being viewed by others
References
Borgstrom F et al (2007) The societal burden of osteoporosis in Sweden. Bone 40(6):1602–1609
Dimai HP et al (2012) Economic burden of osteoporotic fractures in Austria. Health Econ Rev 2(1):12
Hansen L et al (2013) A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos 8(1–2):126
Kanis JA et al (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23(9):2239–2256
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Rizzoli R et al (2010) Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46(2):294–305
The Tromsø Study. Available from: http://www.tromsostudy.com. Accessed 22 Oct 2013
Omsland TK et al (2008) In vivo and in vitro comparison of densitometers in the NOREPOS study. J Clin Densitom 11(2):276–282
Lunar enCore, Supplement til pediatrisk referansedata. 1. revision ed. 2010-Nov: GE Healthcare
Cole TJ et al (2007) Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 335(7612):194
Cole TJ et al (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320(7244):1240–1243
BMI-classes, WHO. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 22 Oct 2013
Koo MM, Rohan TE (1997) Accuracy of short-term recall of age at menarche. Ann Hum Biol 24(1):61–64
Petersen A et al (1988) A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 17(2):117–133
Graff-Iversen S et al (2008) Two short questionnaires on leisure-time physical activity compared with serum lipids, anthropometric measurements and aerobic power in a suburban population from Oslo, Norway. Eur J Epidemiol 23(3):167–174
Lofthus CM et al (2001) Epidemiology of hip fractures in Oslo, Norway. Bone 29(5):413–418
Lofthus CM et al (2008) Epidemiology of distal forearm fractures in Oslo, Norway. Osteoporos Int 19(6):781–786
Rosvold Berntsen GK et al (2000) The Tromso study: determinants of precision in bone densitometry. J Clin Epidemiol 53(11):1104–1112
Oldroyd B, Smith AH, Truscott JG (2003) Cross-calibration of GE/Lunar pencil and fan-beam dual energy densitometers—bone mineral density and body composition studies. Eur J Clin Nutr 57(8):977–987
Crabtree NJ et al (2005) Pediatric in vivo cross-calibration between the GE Lunar Prodigy and DPX-L bone densitometers. Osteoporos Int 16(12):2157–2167
Dorn LD et al (2013) Longitudinal impact of substance use and depressive symptoms on bone accrual among girls aged 11-19 years. J Adolesc Health 52(4):393–399
Kolle E, Torstveit MK, Sundgot-Borgen J (2005) Bone mineral density in Norwegian premenopausal women. Osteoporos Int 16(8):914–920
Kroger H et al (1992) Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner 17(1):75–85
Maynard LM et al (1998) Total-body and regional bone mineral content and areal bone mineral density in children aged 8-18 y: the Fels Longitudinal Study. Am J Clin Nutr 68(5):1111–1117
Júliusson PB et al (2009) Vekstkurver for norske barn. Tidsskr Nor Legeforen 129(4):281–286
Gilsanz V et al (2011) Age at onset of puberty predicts bone mass in young adulthood. J Pediatr 158(1):100–105, 105 e1–2
Deere K et al (2012) Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res 27(9):1887–1895
Arabi A et al (2004) Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone 35(5):1169–1179
Hoiberg M et al (2007) Population-based reference values for bone mineral density in young men. Osteoporos Int 18(11):1507–1514
Moretto de Oliveria MR et al (2011) Bone mineral density in healthy female adolescents according to age, bone age and pubertal breast stage. Open Orthop J 5:324–330
Gracia-Marco L et al (2010) Bone mass and bone metabolism markers during adolescence: the HELENA Study. Horm Res Paediatr 74(5):339–350
Eleftheriou KI et al (2013) Bone structure and geometry in young men: the influence of smoking, alcohol intake and physical activity. Bone 52(1):17–26
Fan B et al (2010) Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int 21(7):1227–1236
Heaney RP et al (2000) Peak bone mass. Osteoporos Int 11(12):985–1009
Dimitri P et al (2012) Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone 50(2):457–466
Clark EM, Ness AR, Tobias JH (2006) Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 91(7):2534–2541
Timpson NJ et al (2009) How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res 24(3):522–533
Javaid MK et al (2011) Growth in childhood predicts hip fracture risk in later life. Osteoporos Int 22(1):69–73
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359(9320):1841–1850
Bielemann RM, Martinez-Mesa J, Gigante DP (2013) Physical activity during life course and bone mass: a systematic review of methods and findings from cohort studies with young adults. BMC Musculoskelet Disord 14:77
Logstein B, Blekesaune A, Almas R (2013) Physical activity among Norwegian adolescents—a multilevel analysis of how place of residence is associated with health behaviour: the Young-HUNT study. Int J Equity Health 12:56
Yoon V, Maalouf NM, Sakhaee K (2012) The effects of smoking on bone metabolism. Osteoporos Int 23(8):2081–2092
Rudang R et al (2012) Smoking is associated with impaired bone mass development in young adult men: a 5-year longitudinal study. J Bone Miner Res 27(10):2189–22197
Wosje KS, Kalkwarf HJ (2007) Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int 18(3):391–400
Berg KM et al (2008) Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 121(5):406–418
Acknowledgements
The authors are grateful to the study participants, the staff at the Centre for Clinical Research and Education, UNN and the Fit Futures administration. The Norwegian Osteoporosis Association supported paediatric software, and the North-Norway Health Authorities funded this work.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winther, A., Dennison, E., Ahmed, L.A. et al. The Tromsø Study: Fit Futures: a study of Norwegian adolescents’ lifestyle and bone health. Arch Osteoporos 9, 185 (2014). https://doi.org/10.1007/s11657-014-0185-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-014-0185-0