Abstract
Objective
To evaluate the efficacy and safety of salvianolate in elderly patients with unstable angina pectoris (UAP).
Methods
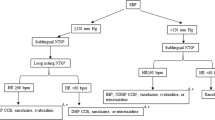
A prospective double-blind randomized placebo-controlled multicenter trial in elderly patients with UAP from 13 third-grade class-A hospitals in China was performed. A total of 318 patients were randomly allocated in a 1:1 ratio to an experimental group (160 patients) and a control group (158 patients). The experimental group was treated with salvianolate for 14 days on the basis of conventional medicine, and the control group was given a placebo for 14 days with the same criteria. Follow-up was lasted 28 days in both groups. The primary endpoint was biweekly frequency of angina pectoris attacks. The secondary endpoints included biweekly dosage of nitroglycerin, the Seattle Angina Questionnaire, angina pectoris severity and duration, myocardial injury markers, high-sensitivity C-reactive protein (hs-CRP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), as well as major adverse cardiovascular events (MACEs). Safety was assessed according to adverse events and serious adverse events.
Results
Baseline characteristics were similar between treatment groups. Compared with those in the control group, the frequency of biweekly angina attacks (2.92 vs . 4.08, P=0.025), the biweekly dosage of nitroglycerin, as well as the severity and duration of angina attacks (P<0.01) were reduced by salvianolate. The Seattle Angina Questionnaire score was also significantly improved in the experimental group than in the control group (P<0.05). No significant differences were observed between the two groups with respect to the incidence of MACEs. Salvianolate was well tolerated.
Conclusions
Salvianolate appear to have efficacy and well tolerated for elderly patients with UAP. [ClinicalTrials.gov identifier: NCT03037047]
Similar content being viewed by others
References
Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction-summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002;40:1366–1374.
Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L, Investigators E. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48:566–575.
Holubkov R, Laskey WK, Haviland A, Slater JC, Bourassa MG, Vlachos HA, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J 2002;144:826–833.
Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schonberger JP, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. New Engl J Med 2001;344:1117–1124.
Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/ non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284:835–842.
De Servi S, Cavallini C, Dellavalle A, Santoro GM, Bonizzoni E, Marzocchi A, et al. Non-ST-elevation acute coronary syndrome in the elderly: treatment strategies and 30-day outcome. Am Heart J 2004;147:830–836.
Jia JY, Lu YL, Li XC, Liu GY, Li SJ, Liu Y, et al. Pharmacokinetics of depside salts from Salvia miltiorrhiza in healthy Chinese volunteers: A randomized, openlabel, single-dose study. Current Therap Res Clin Exp 2010;71:260–271.
Huang J, Yuan M, Ma J, Liu R, Dong Z, Zhao G, et al. Protective effects of salvianolate on contrast-induced nephropathy after primary percutaneous coronary intervention: A prospective multicenter randomized controlled trial. Cardiology 2017;138:169–178.
Li X, Yu C, Sun W, Liu G, Jia J, Wang Y. Simultaneous determination of magnesium lithospermate B, rosmarinic acid, and lithospermic acid in beagle dog serum by liquid chromatography/tandem mass spectrometry. Rapid commun Mass Spectro 2004;18:2878–2882.
Zhang Y, Xie Y, Liao X, Jia Q, Chai Y. A Chinese patent medicine Salvia miltiorrhiza depside salts for infusion combined with conventional treatment for patients with angina pectoris: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2017;25:100–117.
Wu WY, Wang YP. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol Sin 2012;33:1119–1130.
Qi Y, Yu C, Tang L, Li S, Sun X. Patient characteristics associated with treatment response in patients receiving salvianolate injection for stable angina. J Evidence-Based Med 2018;11:83–88.
Zhang D, Wu J, Liu S, Zhang X, Zhang B. Salvianolate injection in the treatment of unstable angina pectoris: A systematic review and meta-analysis. Medicine 2016;95:e5692.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr., et al. 2014 AHA/ACC Guideline for the Management of Patients with Non- ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228.
Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-STElevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1–e157.
Chinese Society of Cardiology of Chinese Medical Association EBoCJoC. Guideline for diagnosis and treatment of patients with unstable angina and non-STsegment elevation myocardial infarction. Chin J Cardiol (Chin) 2007;35:295–304.
Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341.
Saunderson CE, Brogan RA, Simms AD, Sutton G, Batin PD, Gale CP. Acute coronary syndrome management in older adults: guidelines, temporal changes and challenges. Age Ageing 2014;43:450–455.
Fei AH, Cao Q, Chen SY, Wang HR, Wang FL, Pan SM, et al. Salvianolate inhibits reactive oxygen species production in H(2)O(2)-treated mouse cardiomyocytes in vitro via the TGFbeta pathway. Acta Pharmacol Sin 2013;34:496–500.
Meng C, Zhuo XQ, Xu GH, Liu JL. Protection of salvianolate against atherosclerosis via regulating the inflammation in rats. J Huazhong Univ Sci Technol Med Sci (Chin) 2014;34:646–651.
Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovas Qual Outcomes 2009;2:344–353.
Arnold SV, Kosiborod M, Li Y, Jones PG, Yue P, Belardinelli L, et al. Comparison of the Seattle Angina Questionnaire with Daily Angina Diary in the TERISA clinical trial. Circulation: Cardiovas Qual Outcomes 2014;7:844–850.
Drozda J, Jr., Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation 2011;124:248–270.
Dong P, Hu H, Guan X, Ung COL, Shi L, Han S, et al. Cost-consequence analysis of salvianolate injection for the treatment of coronary heart disease. Chin Med 2018;13:28.
Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994;74:1240–1244.
Chen ZW, Xie YM, Liao X, Wang GQ. Systematic review on safety of Salvianolate injection. China J Chin Mater Med 2016;41:3686–3695.
Yan YY, Yang YH, Wang WW, Pan YT, Zhan SY, Sun MY, et al. Post-marketing safety surveillance of the Salvia miltiorrhiza Depside Salt for infusion: a real world study. PloS One 2017;12:e0170182.
Xuan JW, Xu YR, Liu HY, Gao Y, Li LG. A real world cost comparison between Chinese patents with coronary heart disease or angina pectoris using salvianolate and without using Salvianolate: a retrospectve database analysis. J Health Med Economics 2018;4:6.
Acknowledgments
We thank Fudan University professionals for completing the statistical analysis.
Author information
Authors and Affiliations
Contributions
Li XY is the guarantor of this article and contributed to the conception of scope and protocol; provided supervision, coordination and guidance for collaborating, peripheral centers; conceived and implemented the analysis plan, and drafted the initial and final manuscript. Cui H, Gao XW, Lu X, Wu XP, Wang XF, Zheng XQ, Huang K, Liu F, Luo Z, Yuan HS, Sun G, Kong J, Du XH, Liu HY, Zheng J and Zhang WJ were responsible for data acquisition and took responsibility for the integrity of the data and the accuracy of the analysis. All authors have agreed and approved the final manuscript.
Corresponding author
Additional information
Conflicts of Interest
Shanghai Green Valley Pharmaceutical Co. Ltd. (Shanghai, China) provided the salvianolate and placebo. The Shanghai Green Valley Pharmaceutical Co. Ltd. had no participation or influence on the study design, data collection, statistical analysis, and interpretation of results.
Supported by Science and Technology Commission of Shanghai Municipality (15DZ1900300)
Rights and permissions
About this article
Cite this article
Cui, H., Li, Xy., Gao, Xw. et al. A Prospective Randomized Multicenter Controlled Trial on Salvianolate for Treatment of Unstable Angina Pectoris in A Chinese Elderly Population. Chin. J. Integr. Med. 25, 728–735 (2019). https://doi.org/10.1007/s11655-019-2710-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-019-2710-x