Abstract
Objective
To investigate the therapeutic effects of Qingre Quyu Granule (清热祛瘀颗粒, QQG) on the patients with severe carotid stenosis, and to explore the mechanism of it.
Methods
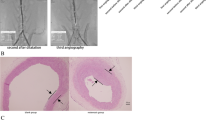
Ninety-six patients with severe carotid stenosis were enrolled in the study and were classified into a QQG group (n=48) and a control group (n=48) randomly using consecutively numbered envelopes. The patients in the QQG group were given QQG and Western medicine, those in the control group were given Western medicine merely, the course of treatment was 16 weeks. All patients went through endarterectomy after treatment. Plaques were subjected to the analysis of CD3, CD68, soluble intercellular adhesion molecule 1 (ICAM-1), matrix metalloprotease-9 (MMP-9), CD40L, tenascin-C, and collagen content lipid content by immunohistochemistry or polarized light analysis.
Results
By the end of experiment, the expressions of CD3, CD68, ICAM-1, MMP9, CD40L and tenascin-C on the plaques were statistically significant lower in the QQG group compared with the control group(P<0.01). The lipid content of the plaque was also significantly lower in the QQG group compared with the control group (P<0.01). The interstitial collagen in the tissue sections of the plaques was also significantly higher in the QQG group in comparison with the control group (P<0.01).
Conclusion
QQG could stabilize carotid artery plaques through inhibiting pro-inflammation factors and restraining the tenascin-C and MMP9 pathway.
Similar content being viewed by others
References
Gualandro DM, Campos CA, Calderaro D, Yu PC, Marques AC, Pastana AF, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: Frequent and dangerous. Atherosclerosis 2012;222:191–195.
Wang Y, Cheng WL, Ke YN, Cai Z, Chen L, Xi Y, et al. Effect of Qingre Quyu Granule on stabilizing plaques in the brachiocephalic artery of Apolipoprote in Edefficient mice. Chin J Integr Med 2010;16:442–447.
Cheng WL, Wang Y, Cai Z, Ke YN, Liu XF, Fan SY, et al. Effect of Qingre Quyu Granule on the vulnerable atherosclerotic plaque of carotid artery in patients with stable coronary artery disease. Chin J Integr Tradit West Med (Chin) 2009;29:1085–1088.
North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711–720.
Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular omeostasis. Circ Res 2012;110:356–369.
Schrijvers DM, De Meyer GR, Martinet W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 2011;31:2787–2791.
Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost 2011;106:827–838.
Katsargyris A, Klonaris C, Tsiodras S, Bastounis E, Giannopoulos A, Theocharis S. Statin treatment is associated with reduced toll-like receptor 4 immunohistochemical expression on carotid atherosclerotic plaques: a novel effect of statins. Vascular 2011;19:320–326.
Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke 2006;37:1923–1932.
Luchtefeld M, Bandlow N, Tietge UJ, Grote K, Pfeilschifter J, Kaszkin M, et al. Angiotensin II type 1-receptor antagonism prevents type II A secretory phospholipase A2-dependentlipid peroxidation. Atherosclerosis 2007;194:62–70.
Fukuda D, Enomoto S, Nagai R, Sata M. Inhibition of reninangiotensin system attenuates periadventitial inflammation and reduces atherosclerotic lesion formation. Biomed Pharmacother 2009;63:754–761.
Kog J, Egashira K, Matoba T, Kubo M, Ihara Y, Iwai M, et al. Essential role of angiotensin II type 1a receptors in the host vascular wall, but not the bone marrow, in the pathogenesis of angiotensin II-induced atherosclerosis. Hypertens Res 2008;31:1791–1800.
Mason RP. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: focus on olmesartan medoxomil. Vasc Health Risk Manag 2011;7:405–416.
Zheng XY. The clinical guiding principle of new drug of traditional Chinese medicine. Beijing: Chinese Medical Science and Technology Press; 2004.
Pedretti M, Rancic Z, Soltermann A, Herzog BA, Schliemann C, Lachat M, et al. Comparative immunohistochemical staining of atherosclerotic plaques using F16, F8 and L19: Three clinical-grade fully human antibodies. Atherosclerosis 2010;208:382–389.
Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res 2011;92:19–28.
Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, et al. Tenascin-C is expressed in macrophagerich human coronary atherosclerotic plaque. Circulation 1999;99:1284–1289.
Siri A, Knäuper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem 1995;270:8650–8654.
Jones PL, Jones FS, Zhou B, Rabinovitch M. Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a beta3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. J Cell Sci 1999;112(Pt 4):435–445.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Capital Medical Development Scientific Research Foundation (No. SF-2007-III-41) and National Nature Science Foundation (No. 81173420)
Rights and permissions
About this article
Cite this article
Wang, Y., Cheng, Wl., Wang, Y. et al. Qingre Quyu Granule (清热祛瘀颗粒) stabilizes plaques through inhibiting the expression of tenascin-C in patients with severe carotid stenosis. Chin. J. Integr. Med. 21, 339–345 (2015). https://doi.org/10.1007/s11655-015-2161-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-015-2161-y