Abstract
Objective
To elucidate the mechanism of Chinese tuina in treating sciatic nerve crush injury, and to detect the levels of tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1), which is thought to play an important role in nerve regeneration.
Methods
Thirty-two adult male Sprague-Dawley rats were subjected to sciatic nerve crush injury and 16 rats (sham-operated group) went through a sham operation. Control group was given no treatment while tuina group received tuina therapy since day 7 post-surgery. Tuina treatment was performed once a day and lasted for 20 days. The sciatic functional index was examined every 5 days during the treatment session. The rats’ gastrocnemius muscles were evaluated for changes in mass and immunohistochemistry techniques were performed to detect the levels of tPA and PAI-1.
Results
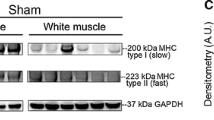
Tuina therapy improved the motor function of sciatic nerve injured rats (P<0.05), however, it did not increase muscle volume (P<0.05). Tuina downregulated the levels of tPA and PAI-1 (P<0.05).
Conclusions
The present study implies that tuina treatment could accelerate rehabilitation of peripheral nerve injury.
Similar content being viewed by others
References
Yu TY, ed. Anmo tuina xue. 3rd ed. Beijing: Peking Union Medical College Press; 2012:3–4.
Jin HZ. Chinese tuina. 1st ed. Shanghai: Publishing House of Shanghai University of Traditional Chinese Medicine; 2002:1–2.
Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol 2011;229:460–470.
Barghash Z, Larsen JO, Al-Bishri A, Kahnberg KE. Degeneration and regeneration of motor and sensory nerves: a stereological study of crush lesions in rat facial and mental nerves. Intern J Oral Maxill Surg 2013;42:1566–1574.
Gigo-Benato D, Russo TL, Geuna S, Domingues NR, Salvini TF, Parizotto NA. Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve 2010;41:685–693.
Martins DF, Mazzardo-Martins L, Gadotti VM, Nascimento FP, Lima DA, Speckhann B, et al. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain 2011;152:2653–2661.
Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol 2013;240:157–167.
Jeon Y, Kim CE, Jung D, Kwak K, Park S, Lim D. Curcumin could prevent the development of chronic neuropathic pain in rats with peripheral nerve injury. Curr Ther Res 2013;74:1–4.
Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 1998;45:116–122.
Lad SP, Nathan JK, Schubert RD, Boakye M. Trends in median, ulnar, radial, and brachioplexus nerve injuries in the United States. Neurosurgery 2010;66:953–960.
Shields RK, Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: effects of longterm soleus electrical stimulation training. Neurorehabil Neural Repair 2007;21:169–179.
Haastert-Talini K, Schmitte R, Korte N, Klode D,Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma 2011;28:661–674.
Wan LD, Xia R, Ding WL. Electrical stimulation enhanced remyelination of injured sciatic nerves by increasing neurotrophins. Neuroscience 2010;169:1029–1038.
Lu MC, Tsai CC, Chen SC, Tsai FJ, Yao CH, Chen YS. Use of electrical stimulation at different current levels to promote recovery after peripheral nerve injury in rats. J Trauma 2009;67:1066–1072.
Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol 2009;219:258–265.
Rochkind S, Geuna S, Shainberg A. Chapter 25: phototherapy in peripheral nerve injury: effects on muscle preservation and nerve regeneration. Int Rev Neurobiol 2009;87:445–464.
Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg 2007;25:137–143.
Câmara CN, Brito MV, Silveira EL, Silva DS, Simões VR, Pontes RW. Histological analysis of low-intensity laser therapy effects in peripheral nerve regeneration in Wistar rats. Acta Cir Bras 2011;26:12–18.
Rochkind S. Phototherapy in peripheral nerve regeneration: from basic science to clinical study. Neurosurg Focus 2009;26:E8.
Hao J, Zhao C, Cao S, Yang S. Electric acupuncture treatment of peripheral nerve injury. J Tradit Chin Med 1995;15:114–117.
Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science 1981;213:1532–1534.
Akassoglou K, Kombrinck KW, Degen JL, Srickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol 2000;149:1157–1166.
Siconolfi LB, Seeds NW. Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci 2001;21:4348–4355.
O’Rourke J, Jiang X, Hao ZF, Cone RE, Hand AR. Distribution of sympathetic tissue plasminogen activator (tPA) to a distant microvasculature. J Neurosci Res 2005;79:727–733.
Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen 1998;6:194–201.
Li WY, Chong SS, Huang EY, Tuan TL. Plasminogen activator/plasmin system: a major player in wound healing? Wound Repair Regen 2003;11:239–247.
Cao C, Lawrence DA, Li Y, von Arnim CA, Herz J, Su EJ, et al. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J 2006;25:1860–1870.
Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, et al. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost 2008;100:1014–1020.
Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 1989;83:129–138.
Funakoshi H, Risling M, Carlstedt T, Lendahl U, Timmusk T, Metsis M, et al. Targeted expression of a multifunctional chimeric neurotrophin in the lesioned sciatic nerve accelerates regeneration of sensory and motor axons. Proc Natl Acad Sci U S A 1998;95:5269–5274.
Liu Q, Luo ZY. Experimental observation of influence of manual manipulations in Chinese massage to temperature of body surface. Shanghai J Tradit Chin Med (Chin) 1999;9:44–45.
Li J, Wei DM, Wen X, Xie WJ. Effect of massage manipulation on blood rheology and microcirculation of adjuvantinduced arthritis of rabbit. Acta Chin Med Pharmacol (Chin) 2010;38:37–39.
J. Chen, J Li. Effects of massotherapy on hemorheology, NO, ET in the patients with cervical spondylotic vertebral arteriopathy. Lishizhen Med Mater Med Res (Chin) 2008;19:2028–2029.
Li ZY, Chen PQ, Yan JT, Liu X, Chen XY, Wu GC. Analgesic effect of tender point kneading on neuralgia in rats. Shanghai J Tradit Chin Med (Chin) 2004;38(5):54–56.
Ji B, Jin HZ, Zhang SN, Shao MX. Effect of four-finger maßsage on β-endorphine and p-substance in patients with protrusion of lumbar intervertebral disc. J Nanjing Tradit Chin Med Univ (Chin) 2007;23:322–324.
Mei XH, Ji Q, Wu JC, Pan F, Wang L, Yu TY. Influences of tuina therapy on nerve growth factor and TrkA receptor of NGF in rats with sciatic nerve injury. J Beijing Univ Tradit Chin Med (Chin) 2013;36:497–500.
Mei XH, Ji Q, Yao BB, Wu JC, LU MQ, Yu TY. Investigation of tuina therapy on NGF and p75NTR of sciatic nerve injury model rats. China J Tradit Chin Med Pharm (Chin) 2013;28:1994–1997.
Yao BB, Mei XH, Wu JC. Study on the influence of massage to sciatic nerve injured rats axoplasmic transport function based on motor protein. J Nanjing Univ Tradit Chin Med (Chin) 2013;27:338–341.
Ma J, Liu J, Yu H, Wang Q, Chen Y, Xiang L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci Lett 2013;547:26–31.
Marcolino AM, Barbosa RI, das Neves LM, Mazzer N, de Jesus Guirro RR, de Cássia Registro Fonseca M. Assessment of functional recovery of sciatic nerve in rats submitted to low-level laser therapy with different fluences. An experimental study: laser in functional recovery in rats. J Hand Microsurg 2013;5:49–53.
De Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 1982;77:634–643.
Varejão AS, Meek MF, Ferreira AJ, Patrício JA, Cabrita AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J Neurosci Methods 2001;108:1–9.
Zou T, Ling CC, Xiao Y. Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J Neuropathol Exp Neurol 2006;65:78–86.
Siconolfi LB, Seeds NW. Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J Neurosci 2001;21:4336–4347.
Acknowledgment
The authors wish to thank YU Yue (The People’s Hospital of Ji County, Tianjin) for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 81373759), Research Fund for the Doctoral Program of Higher Education (No. 20130013110016), Natural Science Foundation of Beijing (No. 7142097)
Rights and permissions
About this article
Cite this article
Pan, F., Yu, Ty., Wong, S. et al. Chinese tuina downregulates the elevated levels of tissue plasminogen activator in sciatic nerve injured Sprague-Dawley rats. Chin. J. Integr. Med. 23, 617–624 (2017). https://doi.org/10.1007/s11655-015-2142-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-015-2142-1