Abstract
Objective
To explore the mechanism of electroacupuncture (EA)-induced cumulative analgesic effects on chronic pain in rats with or without ovariectomy (OVX).
Methods
A total of 110 female Wistar rats were randomized into normal control (n=10), chronic constrictive injury (CCI, n=10), CCI+EA (n=30), OVX+CCI (n=30), and OVX+CCI+EA (n=30) groups. Each of the latter 3 groups was further divided into 2 days (2 d), 2 weeks (2 W) and 3 weeks (3 W) subgroups, respectively (n=10 in each subgroup). The CCI pain model was established by ligature of the right sciatic nerve, and the memory impairment model duplicated by OVX. The paw withdrawal latency (PWL, pain threshold) of the bilateral footplates was detected by radiant heat irradiation, and the bilateral difference in PWL (PWLD) was used to evaluate changes in the pain reaction. Morris water maze test was conducted for evaluating the rats’ learning-memory ability. EA was applied to bilateral Zusanli (ST36) and Yanglingquan (GB34) for 2 d, 2 W and 3 W, respectively. Pituitary and hypothalamic β-endorphin (EP) and adrenocorticotrophic hormone (ACTH) contents were detected by immunoradioassay.
Results
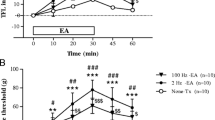
Compared with the CCI group, PWLD of the CCI+EA-3 W group decreased signifificantly (P<0.05). Compared with the OVX+CCI group, PWLD of the OVX+CCI+EA-3 W group was lowered considerably (P<0.05), but the value was markedly higher than its basal value and those of the normal control and CCI+EA groups (P<0.05). In comparison with the sham-OVX group, the escape latency, swimming distance (SD) in the target quadrant and total SD were increased remarkably in the OVX group (P<0.05), while the number of target platform crossings was decreased signifificantly (P<0.05), suggesting an impairment of the OVX rats’ learning-memory ability. In simple CCI rats, both β-EP and ACTH contents of the pituitary increased markedly (P<0.05), and those of the hypothalamus decreased obviously compared to the normal control group (P<0.05). After EA, pituitary and hypothalamic ACTH levels were signifificantly lowered at 2 d and hypothalamic ACTH and β-EP contents increased obviously at 3 W in comparison with the CCI group (P<0.05). In OVX+CCI rats, following EA, pituitary β-EP contents at 2 d, 2 W and 3 W, and hypothalamic β-EP and ACTH contents at 2 W and hypothalamic ACTH levels at 3 W increased signifificantly (P<0.05), but hypothalamic β-EP level at 3W decreased markedly (P<0.05). The effects of repeated EA in lowering pituitary ACTH and raising hypothalamic β-EP and ACTH levels disappeared after OVX+CCI.
Conclusions
Repeated EA has a cumulative analgesic effect, which is closely associated with its effects in regulating pituitary and hypothalamicβ-EP and ACTH levels. OVX may weaken the analgesic effect of EA by affecting hypothalamic-pituitary axis activity.
Similar content being viewed by others
References
Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain 2000;86:217–225.
Tsao JCI, Meldrum M, Bursch B, Jacob MC, Kim SC, Zeltzer LK. Treatment expectations for CAM interventions in pediatric chronic pain patients and their parents. Evid Based Complment Altern Med 2005;2:521–527.
Dong ZQ, Xie H, Ma F, Li WM, Wang YQ, Wu GC. Effects of electroacupuncture on expression of somatostatin and preprosomatostatin mRNA in dorsal root ganglions and spinal dorsal horn in neuropathic pain rats. Neurosci Lett 2005;385:189–194.
Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: the effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Phys Ther 2006;86:698–709.
Jana Moore Capristo, L. Ac. Moore acupuncture. The cumulative effect: http://www.mooreacupuncture.com/thecumulativeeffect.html (November 6, 2008).
Takahashi H. Effects of scalp acupuncture and auricular therapy on acute herpetic pain and postherpetic neuralgia: a case series. Med Acupunct 2007;19:113–120.
Cui KM, Li WM, Gao X, Chung K, Chung JM, Wu GC. Electroacupuncture relieves chronic visceral hyperalgesia in rats. Neurosci Lett 2005;376:20–23.
Wang JY, Chen SP, Li YH, Meng FY, Gao YH, Liu JL. Observation on the accumulative analgesic effect of electroacupuncture and the expression of protein kinase A in hypothalamus and hippocampus in chronic pain or/and ovariectomized rats. Acupunct Res (Chin) 2008;33(2):80–87.
Luo F. A study on the cumulative effect of repeated electroacupuncture on chronic pain. Prog Physiol Sci (Chin) 1996;27:241–244.
Yokoyama M, Sun X, Oku S, Taga N, Sato K, Mizobuchi S, et al. Comparison of percutaneous electrical nerve stimulation with transcutaneous electrical nerve stimulation for long-term pain relief in patients with chronic low back pain. Anesth Analg 2004;98:1552–1556.
Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;26:17–22.
Masala A, Santa G, Alagna S, Zolo TA, Rovasio PP, Rassu S. Suppression of electroacupuncture (EA)-induced β-endorphin and ACTH release by hydrocortisone in man. Absence of effects on EA-induced anaesthesia. Acta Endoc rinol 1983;103:469–472.
Dong ZQ, Ma F, Xie H, Wang YQ, Wu GC. Downregulation of GFR alpha-1 expression by antisense oligodeoxynucleotide attenuates electroacupuncture analgesia on heat hyperalgesia in a rat model of neuropathic pain. Brain Res Bull 2006;69:30–36.
Guo HF. Comparative study on low- and high-frequency electroacupuncture evoked expressions of c-fos/c-jun and opiod-peptide and their relationship. Prog Physiol Sci (Chin) 1996;27:135–139.
Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 2008;63:119–123.
Tang AC, Reeb BC, Romeo RD, McEwen BS. Modification of social memory, hypothalamic-pituitary-adrenal axis, and brain asymmetry by neonatal novelty exposure. J Neurosci 2003;23:8254–8260.
Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci 2006;79:1553–1560.
Hajszan T, Hoyk Z, Garcia-Segura LM, Parducz A. Effects of estradiol and DHEA on morphological synaptic plasticity. In: Neuroactive steroids in brain function, behavior and neuropsychiatric disorders. Springer Netherlands; 2008: 171-185.
Matsumoto A, Arai Y. Neuronal plasticity in the deafferented hypothalamic arcuate nucleus of adult female rats and its enhancement by treatment with estrogen. J Comp Neurol 2004;197:197–205.
Bennet GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87–107.
Nunez J. Morris water maze experiment. http://www.jove.com/index/details.stp?ID=897.
Lumb BM. Hypothalamic and midbrain circuitry that distinguishes between escapable and inescapable pain. News Physiol Sci 2004;19:22–26.
Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci USA 1978; 75:1591–1595.
Pelletier G, Désy L. Localization of ACTH in the human hypothalamus. Cell Tissue Res 1979;196:525–530.
Bowen R. Adrenocorticotropic Hormone (ACTH, corticotropin). http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/hypopit/acth.html. 1998
Bianch M, Sotgui ML, Manfredi B, Sacerdote P. Peripheral mononeuropathy affects hypothalamic and splenocyte betaendorphin levels but not immune function in the rat. Brain Res Bull 1996;40:269–272.
Millan MJ, Millan MH, Czonkowski A, Höllt V, Pilcher CW, Herz A, et al. A model of chronic pain in the rat: response of multiple opioid systems to adjuvant-induced arthritis. J Neurosci 1986;6:899–906.
Höllt V, Przewocki R, Haarmann I, Almeida OF, Kley N, Millan MJ, et al. Stress-induced alterations in the levels of messenger RNA coding for proopiomelanocortin and prolactin in rat pituitary. Neuroendocrinology 1986; 43:277–282.
Zhang LF, Li XH, Zheng Y, Li JD, Yan J, Zhu WL, et al. Effect of electroacupuncture at different acupoint on content of serum interleukin-2 and beta-endorphin in different tissue of adjuvant bondage model rats. Chin J Clin Rehabil (Chin) 2005;9:142–145.
Gameiro GH, Gameiro PH, Andrade Ada S, Pereira LF, Arthuri MT, Marcondes FK, et al. Nociception- and anxietylike behavior in rats submitted to different periods of restraint stress. Physiol Behav 2006;87:643–649.
Liu ZP, Li WX, Yu P, Lu CQ, Chen JS. Effect of compound recipe of Chinese materia medica with estrogen-like effect on estrogen receptor and β-EP level in ovariectomized rats. Acta Acad Med Milit Tertiae (Chin) 2004;26: 1261–1264.
Chen J, Liu GP, Zhou CY. Involvement of hypothalamic β-EP and POMC mRNA expression in the after-effect of acupuncture analgesia. Acupunct Res (Chin) 2004;29:5–9.
Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 2006;88:67–76.
Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain 2005;118:243–253.
Liu JL, Chen SP, Gao YH, Meng FY. Comparison of analgesic effects of electroacupuncture at different strengths and different frequencies. Acupunct Res (Chin) 2006;31:280–285.
Pang Y, Li XC, Li HD, Xiong Y, Gong FY. Study of the effects of electroacupuncture and morphine on cerebral content of ACTH and pain response in rats with chronic pain. Acta Acad Med Milit Tertiae (Chin) 2000;22:720–723.
Liang FR, Liu YX, Luo R, Zhao JL, Yu SG, Xia XH, et al. Observation on the after-effect of different needling techniques on hypothalamic β-EP level in acute adjuvant-induced arthritis rats. China Acu-Moxibust (Chin) 2004; 24:782–784.
Chen BY. Acupuncture normalizes dysfunction of hypothalamic-pituitary-ovarian axis. Acupunct Electrother Res 1997;22(2):97–108.
Wei YF, Liu YL, Zhang SH, Wang ZO, Liu Y, Wang HC, et al. Effect of electroacupuncture on plasma estrin and bone mineral density in ovariectomized rats. Acupunct Res (Chin) 2007;32:38–41.
Zhao H, Tian Z, Feng Y, Chen B. Circulating estradiol and hypothalamic corticotrophin releasing hormone (CRH) increased along with time after ovariectomy in rats: effects of electroacupuncture. Neuropeptides 2005; 39:433–438.
Tian SJ, Yin L, Sun JP, Tian QH, Zu YQ, Zheng Y, et al. Effect of acupuncture on the expression of choline acetyltransferase mRNA in the brain of ovarietomized rats. Acta Physiol Sin (Chin) 2004;56:498–502.
Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology 1991;129:3180–3186.
Wang W, Jiang MC, Yin L, Tian SJ. Effect of electroacupuncture on expression of estrogen receptors mRNA in the brain of ovariectomized rats. Chin J Rehabil Theor Pract (Chin) 2007;13:233–236.
Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem 2004;82:230–242.
Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem 2003;80:194–210.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 30472241, No. 90709031) and the Major State Basic Research Development Program of China (973 Program, No. 2007CB512505)
Rights and permissions
About this article
Cite this article
Liu, Jl., Chen, Sp., Gao, Yh. et al. Effects of repeated electroacupuncture on β-endorphin and adrencorticotropic hormone levels in the hypothalamus and pituitary in rats with chronic pain and ovariectomy. Chin. J. Integr. Med. 16, 315–323 (2010). https://doi.org/10.1007/s11655-010-0503-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-010-0503-3