Abstract
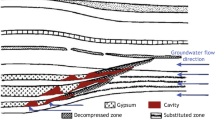
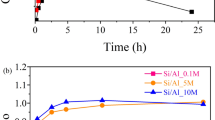
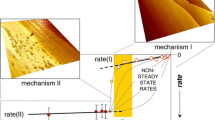
Kyanite is an important and slow-dissolving mineral. Earlier work has measured its dissolution rate at high temperature and acidic pH, but experimental measurements at low temperature and near neutral pH were lacking. The rate equation by Palandri and Kharaka (A compilation of rate parameters of water–mineral interaction kinetics for application to geochemical modeling. US Geological Survey, Open File Report 2004-1068, 2004) indicates that the rate of kyanite dissolution at room temperature and near neutral pH is on the order of 10−17 mol m−2 s−1, orders of magnitudes slower than most common silicate minerals such as albite and quartz. This study used an externally-stirred mixed-flow reactor, which allows high solid:solution ratios, to measure the dissolution rate of kyanite at 0–22 °C and pH of 3.5–7.5. The measured dissolution rate of kyanite is 4.6–7.6 × 10−13 mol m−2 s−1 at 22 °C, and the apparent activation energy is 73.5 kJ mol−1. This dissolution rate is close to the rate of quartz dissolution and four orders of magnitude faster than the prediction by rate equation of Palandri and Kharaka (2004).
Based on our new experimental data, we recommend the following rate equation for modeling the dissolution of kyanite at ambient temperatures.
where k = 5.08 × 10−13 mol m−2 s−1, and Ea = 73.5 kJ mol−1. Review of literature data (Carroll in The dissolution behavior of corundum, kaolinite, and andalusite: a surface complex reaction model for the dissolution of aluminosilicate minerals in diagenetic and weathering environs. Dissertation, Northwestern University, 1989) led to a recommended rate equation for andalusite as for T = 25 °C and pH = 2–10:
where k1 = 4.04 × 10−10 mol m−2 s−1, k2 = 7.95 × 10−10 mol m−2 s−1, k3 = 1.01 × 10−17 mol m−2 s−1, n1 = 1.2 and n3 = − 0.6.








Similar content being viewed by others
References
Apps J, Spycher N (2004) Data qualification for thermodynamic data used to support THC calculations. ANL-NBS-HS-000043 REV 00, Bechtel SAIC Company, LLC, Las Vegas, NV. DOC.20041118.0004
Brantley SL (2008) Kinetics of mienral dissolution. In: Brantley SL, Kubicki JD, White AF (eds) Kinetics of water–rock interaction. Springer, New York, pp 151–210
Carroll SA (1989) The dissolution behavior of corundum, kaolinite, and andalusite: a surface complex reaction model for the dissolution of aluminosilicate minerals in diagenetic and weathering environs. Dissertation, Northwestern University
Dryden L, Dryden C (1946) Comparative rates of weathering of some common heavy minerals. J Sediment Res 16:91–96
Ganor J, Lu P, Zheng Z, Zhu C (2007) Bridging the gap between laboratory measurements and field estimations of silicate weathering using simple calculations. Environ Geol 53:599–610
Govett G (1961) Critical factors in the colorimetric determination of silica. Anal Chim Acta 25:69–80
Gudbrandsson S, Wolff-Boenisch D, Gislason SR, Oelkers EH (2014) Experimental determination of plagioclase dissolution rates as a function of its composition and pH at 22 °C. Geochim Cosmochim Acta 139:154–172
Helgeson HC, Delany JM, Nesbitt HW, Bird DK (1978) Summary and critique of the thermodynamic behavior of aqueous electrolytes at high pressure and temperature. Am J Sci 787:1–229
Helgeson HC, Kirkham DH, Flowers GC (1981) Theoretical prediction of the thermodynamic behavior of aqueous electrolytes by high pressures and temperatures; IV, Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 degrees C and 5kb. Am J Sci 281:1249–1516
Holland T, Powell R (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J Metamorph Geol 29:333–383
Marini L (2006) Geological sequestration of carbon dioxide: thermodynamics, kinetics, and reaction path modeling. Elsevier, Amsterdam
Oelkers EH, Helgeson HC (1990) Triple-ion anions and polynuclear complexing in supercritical electrolyte solutions. Geochim Cosmochim Acta 54:727–738
Oelkers EH, Schott J (1999) Experimental study of kyanite dissolution rates as a function of chemical affinity and solution composition. Geochim Cosmochim Acta 63:785–797
Palandri JL, Kharaka YK (2004) A compilation of rate parameters of water–mineral interaction kinetics for application to geochemical modeling. US Geological Survey, Open File Report 2004-1068
Parkhurst DL, Appelo C (2013) Description of input and examples for PHREEQC version 3—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US geological survey techniques and methods, book 6, 497
Rimstidt JD (1997) Quartz solubility at low temperatures. Geochim Cosmochim Acta 61:2553–2558
Rimstidt JD (2014) Geochemical Rate models: an introduction to geochemical kinetics. Cambridge University Press, Cambridge
Rimstidt JD, Zhang Y, Zhu C (2016) Rate equations for sodium catalyzed amorphous silica dissolution. Geochim Cosmochim Acta 195:120–125
Shock EL, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000 °C. Geochim Cosmochim Acta 52:2009–2036
Shock EL, Helgeson HC, Sverjensky DA (1989) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: standard partial molal properties of inorganic neutral species. Geochim Cosmochim Acta 53:2157–2183
Shock EL, Oelkers EH, Johnson JW, Sverjensky DA, Helgeson HC (1992) Calculation of the thermodynamic properties of aqueous species at high pressures and temperatures. Effective electrostatic radii, dissociation constants and standard partial molal properties to 1000 °C and 5 kbar. J Chem Soc Faraday Trans 88:803–826
Shock EL, Sassani DC, Willis M, Sverjensky DA (1997) Inorganic species in geologic fluids: correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim Cosmochim Acta 61:907–950
Stefansson A, Gíslason SR (2001) Chemical weathering of basalts, Southwest Iceland: effect of rock crystallinity and secondary minerals on chemical fluxes to the ocean. Am J Sci 301:513–556
Su C, Harsh JB (1994) Gibbs free energies of formation at 298 K for imogolite and gibbsite from solubility measurements. Geochim Cosmochim Acta 58:1667–1677
Su CM, Harsh JB (1998) Dissolution of allophane as a thermodynamically unstable solid in the presence of boehmite at elevated temperatures and equilibrium vapor pressures. Soil Sci 163:299–312
Sverjensky DA, Shock EL, Helgeson HC (1997) Prediction of the thermodynamic properties of aqueous metal complexes to 1000 °C and 5 kb. Geochim Cosmochim Acta 61:1359–1412
Tagirov B, Schott J (2001) Aluminum speciation in crustal fluids revisited. Geochim Cosmochim Acta 65:3965–3992
Tanger JC, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures; revised equations of state for the standard partial molal properties of ions and electrolytes. Am J Sci 288:19–98
Velbel MA (1999) Bond strength and the relative weathering rates of simple orthosilicates. Am J Sci 299:679–696
White A, Blum AE, Schulz MS, Bullen TD, Harden JW, Peterson ML (1996) Chemical weathering rates of a soil chronosequence on granitic alluvium: I. Quantification of mineralogical and surface area changes and calculation of primary silicate reaction rates. Geochim Cosmochim Acta 60:2533–2550
Yang L, Steefel CI (2008) Kaolinite dissolution and precipitation kinetics at 22 °C and pH 4. Geochim Cosmochim Acta 72:99–116
Zhu C, Lu P, Zheng Z, Ganor J (2010) Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems: 4. Numerical modeling of kinetic reaction paths. Geochim Cosmochim Acta 74:3963–3983
Zimmer K, Zhang Y, Lu P, Chen Y, Zhang G, Dalkilic M, Zhu C (2016) SUPCRTBL: a revised and extended thermodynamic dataset and software package of SUPCRT92. Comput Geosci 90:97–111
Acknowledgements
Financial support of this research was provided by the U.S. NSF Grant EAR-1225733 to Dr. Chen Zhu, and Indiana University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Rimstidt, D.J., Huang, Y. et al. Kyanite far from equilibrium dissolution rate at 0–22 °C and pH of 3.5–7.5. Acta Geochim 38, 472–480 (2019). https://doi.org/10.1007/s11631-019-00347-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-019-00347-9