Summary
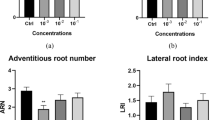
Lignosulfonates (LIGNs) are low-cost by-products from the paper industry and are already commercialized as fertilizers. Because earlier laboratory and glasshouse assays had shown a beneficial effect of LIGNs on rooting and general plant vigor, their incorporation in several plant tissue culture types was examined here. The present assays indicated that well-chosen concentrations of LIGNs, whether they were chelated with Ca or Fe, stimulated growth of a normal and an habituated sugarbeet callus, improved multiplication rate and vigor of a shoot-proliferating poplar cluster, and increased the rooting percentage of holly, ginseng, and poplar shoots. Complementing the exogenous rooting auxin with LIGNs enhanced the increases of endogenous levels of indoleacetic acid and its aspartate conjugate in the basal parts of poplar shoots at the rooting inductive phase. Although LIGNs exerted some effects in the absence of the growth regulators, they could not replace them. Their possible mode of action is discussed.
Similar content being viewed by others
References
Chraibi, B. K.; Latche, A.; Roustan, J. P.; Fallot, J. Stimulation of shoot regeneration from cotyledons of Helianthus annuus by the ethylene inhibitors, silver and cobalt. Plant Cell Rep. 10:204–207; 1991.
Darvill, A.; Augur, C.; Bergmann, C. Oligosaccharins-oligosaccharides that regulate growth development and defence responses in plants. Glycobiology 2:181–198; 1992.
Dumas, E.; Monteuuis, O. In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tissue Organ Cult. 40:231–235; 1995.
Galston, A. W.; Kaur-Sawhney, R. Polyamines as endogenous growth regulators. In: Davies, P. J., ed. Plant hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995:158–178.
Gaspar, T.; Kevers, C. Cobalt prevention of vitrification process in carnation. Plant Physiol. 77(Suppl.):13; 1985.
Gaspar, T.; Kevers, C.; Hausman, J. F. Indissociable chief factors in the inductive phase of adventitious rooting. In: Altman, A.; Waisel, Y., ed. Biology of root formation and development. New York and London: Plenum Press; 1997:55–64.
Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.; Thorpe, T. A. Plant hormones and plant growth regulators in plant tissue cultures. In Vitro Cell. Dev. Biol. 32:272–289; 1996.
Gatineau, F.; Fouche, J. G.; Kevers, C.; Hausman, J. F.; Gaspar, T. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol. Plant. 39:131–137; 1997.
George, E. Plant propagation by tissue culture. Part 1. The technology. Edington: Exegetics Ltd.; 1993.
Gross, D.; Parthier, B. Novel natural substances acting in plant growth regulation. J. Plant Growth Regul. 13:93–114; 1994.
Hausman, J. F.; Kevers, C.; Gaspar, T. Auxin-polyamine interaction in the control of the rooting inductive phase of poplar shoots in vitro. Plant Sci. 110:63–71; 1995.
Kevers, C.; Coumans, M.; De Greef, W.; Jacobs, M.; Gaspar, T. Organogenesis in habituated sugarbeet callus: auxin content and protectors, peroxidase pattern and inhibitors. Z. Pflanzenphysiol. 101:79–87; 1981.
Martin-Tanguy, J.; Aribaud, M.; Carré, M.; Gaspar, T. ODC-mediated biosynthesis and DAO-mediated catabolism of putrescine involved in rooting by leaf explants of Chrysanthellum morifolium Ramat in vitro. Plant Physiol. Biochem. 35:595–602; 1997.
Misson, J. P.; Boxus, P.; Coumans, M.; Giot-Wirgot, P.; Gaspar, T. Rôle du charbon de bois dans les milieux de culture de tissus végétaux. Meded. Fac. Landbouwwet. Rijksuniv. Gent 48/4:1151–1157; 1983.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473–497; 1962.
Nordström, A. C.; Eliasson, L. Levels of endogenous indole-3-acetic acid and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings. Physiol. Plant. 82:599–605; 1991.
Pierpoint, W. S. Salicylic acid and its derivatives in plants: medicines, metabolites and messenger molecules. Adv. Bot. Res. 20:163–235; 1994.
Sembdner, G.; Parthier, B. The biochemistry and the physiological and molecular actions of jasmonates. Ann. Rev. Plant Physiol. Mol. Biol. 44:459–489; 1993.
Soteras, G. Mode d’action des lignosulfonates de fer chez les végétaux. Isolement de la molécule active. Doctoral thesis. Université de Montpellier II. 1994; 176 p.
Telysheva, G.; Lebedeva, G.; Davidthshik, L.; Gross, O.; Viesturs, U. A novel silica-containing preparation for environmental protection. International Workshop and Young Scientists’ School, Bioprocess Engineering. 1994a; p. 4.
Telysheva, G.; Lebedeva, G.; Viesturs, U. Silicon-containing fertilizers on the base of biomass waste. In: Hall, D. O.; Grass G.; Scheer, H., ed. Biomass for energy and industry. Brussels and Luxemburg: Ponte Press; 1994b:584–588.
Telysheva, G.; Lebedeva, G.; Zaimenko, N.; Grivinya, D.; Dizhbite, T.; Virzina, O. Novel ligno-silicon products promoting root system development. In: Altman, A.; Waisel, Y., ed. Biology of root formation and development. New York and London: Plenum Press; 1997:92–93.
Telysheva, G.; Lebedeva, G.; Zaimenko, N.; Viesturs, U. New lignosilicon fertilizers and their action on soil biota. International symposium, Soil Decontamination Using Biological Processes. Karlsruhe, Deutschland; 1992:525–530.
Van der Krieken, W. M.; Kodde, J.; Visser, M. H.; Tsardakas, D.; Blaakmeer, A.; de Groot, K.; Leegstra, L. Increased induction of adventitious rooting by slow release auxins and elicitors. In: Altman, A.; Waisel, Y., ed. Biology of root formation and development. New York and London: Plenum Press; 1997:95–104.
Vasil, I. K.; Thorpe, T. A. Plant cell and tissue culture. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994.
Vieitez, A. M.; Barciela, J.; Ballester, A. Propagation of Camellia japonica cv. alba plena by tissue culture. J. Hortic. Sci. 64:177–182; 1989.
Yamashita, T. T., inventor. Method and composition for promoting and controlling growth of plants. US patent 5549729. 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kevers, C., Soteras, G., Baccou, J.C. et al. Lignosulfonates: Novel promoting additives for plant tissue cultures. In Vitro Cell.Dev.Biol.-Plant 35, 413–416 (1999). https://doi.org/10.1007/s11627-999-0057-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11627-999-0057-2