Abstract
The medicinal plant Hyoscyamus reticulatus L. is a rich source of hyoscyamine and scopolamine, the tropane alkaloids. The use of hairy root cultures has focused significant attention on production of important metabolites such as stable tropane alkaloid production. Elicitation is an effective approach to induce secondary metabolite biosynthetic pathways. Hairy roots were derived from cotyledon explants inoculated with Agrobacterium rhizogenes and elicited by iron oxide nanoparticles (FeNPs) at different concentrations (0, 450, 900, 1800, and 3600 mg L−1) for different exposure times (24, 48, and 72 h). The highest hairy root fresh and dry weights were found in the medium supplemented with 900 mg L−1 FeNPs. Antioxidant enzyme activity was significantly increased in induced hairy roots compared to non-transgenic roots. The highest hyoscyamine and scopolamine production (about fivefold increase over the control) was achieved with 900 and 450 mg L−1 FeNPs at 24 and 48 h of exposure time, respectively. This is the first report of the effect of FeNP elicitor on hairy root cultures of a medicinal plant. We suggest that FeNPs could be an effective elicitor in hairy root cultures in order to increase tropane alkaloid production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyoscyamus reticulatus L. (belonging to Solanaceae family) is one of the most important medicinal plants in South-west Asia, Egypt, Iran, and Turkey (Madani et al. 2015). Hyoscyamus species are the main source of tropane alkaloids, especially scopolamine and hyoscyamine, which are commonly exploited in folk medicine. Due to the complicated chemical formulation of hyoscyamine and scopolamine, their synthetic production is too expensive and so, in practice, they are obtained from Solanaceae plants. They are normally produced in fresh root cells and transported to the aerial plant fragments (Ghorbanpour et al. 2015). Agrobacterium rhizogenes-induced genetically transformed root cultures in many Solanaceous species have revealed their potential for fast production of biomass with high contents of tropane alkaloids (Jouhikainen et al. 1999). For increased secondary metabolite production from medicinal plants, many approaches have been explored (Sharafi et al. 2013a, b; Mirzaee et al. 2016), such as selection of high yielding cell lines, growth media adaptation, elicitation, precursor feeding, large scale culture in bioreactor systems, hairy root culture, plant cell immobilization, and biotransformation. The generation of noteworthy pharmaceutical secondary metabolites in plant cultures based on modern techniques such as tissue culture or genetic transformation methods is an alternate method compared to the extraction from roots. Also, genetic engineering has become an interesting approach for manipulating and revealing regulatory aspects of alkaloid biosynthesis. Development of efficient protocols for induction of hairy roots from some medicinal plants was established in our laboratory by different strains of A. rhizogenes (Sharafi et al. 2013 a; Sharafi et al. 2014 a, b; Valimehr et al. 2014). Elicitation is an effective method for improving the low yields of medicinal plants’ secondary metabolite production. Elicitors are chemicals or biological factors which can induce physiological and morphological reactions and secondary metabolite enhancement. The uses of biotic and abiotic elicitors in hairy root cultures are the most suitable approach for increasing the productivity. Prior studies have described enhancement of secondary metabolite production by different elicitors in hairy root cultures of medicinal plants. Artemisinin production was increased from 1.67 mg to 2.86 mg g−1 dry wt in hairy root cultures of Artemisia annua using 900 mg L−1 Ag-SiO2 core-shell nanoparticles (Zhang et al. 2013). In another study on Anisodus luridus hairy root cultures, the scopolamine efflux reached to 6.2 times comparing to the non-elicitated roots achieved by adding acetylsalicylic acid (ASA) as a chemical elicitor (Qin et al. 2014). Scopolamine is synthesized from hyoscyamine by moderation of 6β-hydroxyhyoscyamine (Fig. 1) (Zhang et al. 2013). Tropane alkaloid production has been elicited in hairy root culture of solanaceous plants such as Brugmansia candida (Pitta-Alvarez et al. 2000), Anisodus acutangulus (Kai et al. 2012), and Hyoscyamus niger (Jaremicz et al. 2013). CaCl2 and hemicellulase can increase the intracellular hyoscyamine and scopolamine accumulation, release, and production in B. candida hairy roots (Pitta-Alvarez et al. 2000). Elicitation of suspension-cultured Corylus avellana L. cells by 5 ppm silver nanoparticles led to enhanced taxol production (Jamshidi et al. 2014). The atropine yield in hairy root cultures of Datura metel induced with nanosilver was increased to 1.147-, 1.117-, and 2.42-fold in comparison to the control samples after 12, 24, and 48 h of treatment, respectively (Shakeran et al. 2015). In Hypericum perfuratum cell suspension cultures, production of hypericin and hyperforin was induced significantly by zinc and iron nanooxides (Sharafi et al. 2013a, b). The highest content of glycyrrhizin was observed in Glycyrrhiza glabra seedlings after elicitation by CuO and ZnO nanoparticles (Oloumi et al. 2015).
Reactive oxygen species are produced by different physicochemical and biochemical reactions. Elicitation with different elicitors may lead to oxidative stress induction. Generally, plants are protected against oxidative stresses by means of a wide range of radical scavenging systems such as antioxidative enzymes like peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT), as well as non-enzymatic compounds (Hatami and Ghorbanpour 2014). Oxidative tension is a general response related to all stresses leading to various secondary responses, such as secondary metabolite generation. Iron oxide nanoparticles diameters are between about 1 and 100 nm. Iron oxide nanoparticles are magnetite, either Fe3O4 or γ-Fe2O3. Because of their paramagnetic attributes and their possible usage in many fields, they have attracted research interest (Sharafi et al. 2013a, b).
Nanomaterials can promote some metabolism and reveal physiological answers but the underlying mechanisms are unknown (Hatami and Ghorbanpour 2014). To the best of our knowledge, no previous study has surveyed the influence of iron oxide nanoparticles as abiotic elicitor on enhancement of hyocyamine and scopolamine productivity in hairy root culture of H. reticulatus L. The main goal of this study is the evaluation of the antioxidant activity, growth, and production of hyoscyamine and scopolamine by elicitation with iron oxide nanoparticles at different concentrations and exposure times in hairy root culture of H. reticulatus L.
Materials and Methods
Plant materials
Seeds of H. reticulatus were provided by Pakan Bazr Company, Isfahan, Iran. H. reticulatus seeds were surface sterilized in 70% (v/v) ethanol and 10% (v/v) NaOCl and then washed three times in sterile water. Afterward, seeds were cultured in MS medium supplemented with 3% (w/v) sucrose, 7.2 g L−1 agar (Duchefa, Haarlem, Netherlands), and 0.1 g L−1 myo-inositol (Duchefa, Netherlands). One week after germinating, cotyledons were isolated as explants.
Hairy root induction and culture
The explants (cotyledons) were infected with A. rhizogenes strain A7 and incubated in the dark on hormone free MS medium supplemented with 3% (w/v) sucrose, 7.2 g L−1 agar, and 0.1 g L−1 myo-inositol and after 48 h transferred to the same medium supplemented with 200 mg L−1 cefotaxim. After 2 weeks, hairy roots were induced and observed. They were sub-cultured every 10 d and after three passages transferred to antibiotic free MS medium. The cultures were transferred to 250 mL Erlenmeyer flasks (shaken at 120 rpm at 25°C in darkness) containing 30 mL hormone-free liquid MS medium and sub-cultured every 2 weeks.
Polymerase chain reaction analysis
Total DNA was extracted from transformed and non-transformed roots using DNA isolation kit (Fermentas Vilnius, Germany). PCR analysis with specific primers of rol B gene was performed. The primers designed to amplify rol B were 5’-tggatcccaaattgctattccacga-3′and 5’-ttaggcttctttcttcaggtttactgcagc-3′. The PCR reactions contained, in a final volume of 20 μL of 1 × PCR buffer, 3 mM MgCl2, 1 mM of each dNTP (Fermentas Co.), 0.4 μM of each specific primer, 1 U of Taq DNA polymerase (Fermentas Co.), and 20 ng genomic DNA or 10 ng pRi plasmid DNA used as positive control. The PCR conditions were 94°C (5 min), 30 cycles of three steps [94°C (1 min), 58°C (1 min), and 72°C (30 s)], and 72°C (10 min) for final extension. PCR products were revealed following electrophoresis on 1% agarose under UV trans-illuminator.
Elicitor preparation and elicitation
Iron oxide (Fe3O4) nanoparticle solution was provided by Nanozaino Co., Tehran, Iran. To investigate the influence of iron oxide nanoparticles (FeNPs), different concentrations of this elicitor (0, 450, 900, 1800, and 3600 mg L−1) were added to MS culture media (pH = 5.8 before autoclaving) of 10-days-old hairy roots of H. reticulatus. Hairy root culture was induced with FeNPs for 24, 48, and 72 h and then transferred to elicitor-free MS culture medium fortified with 3% (w/v) sucrose, 7 g L−1 agar, and 100 mg L−1 myo-inositol for growth and production of tropane alkaloids. Hairy roots were harvested after a week, air-dried, and milled for extraction of alkaloid.
Alkaloid extraction
Alkaloid extraction was performed as described in Kamada et al. (1986). Briefly, 500 mg powdered sample was diluted with 10 mL solvent containing CHCl3/MeOH/25% (w/v) NH4OH (15:5:1 v/v/v) per 100 mg dry sample and sonicated for 10 min, kept at room temperature (1 h), and then filtered. The residue was washed twice with 1 mL of CHCl3 and dried. Five milliliters of CHCl3 and 2 mL of 1 N H2SO4 were added to the residue and mixed. The H2SO4 phase was adjusted to pH 10 with 28% (w/v) NH4OH in an ice bath and extracted once with 2 mL and twice with 1 mL of CHCl3. The combined aqueous extracts were dried over anhydrous Na2SO4, and then, the residue was washed with 1 mL of CHCl3. After evaporation, the extract was dissolved in 1–2 mL MeOH and subjected to GC-MS analysis. GC analysis was performed on a Hewlett–Packard (HP, Palo Alto, CA) HP 7890A. GC-MS analysis was based on Gharari et al. (2016) method.
Enzyme assay
Enzyme extraction was performed as in Kang and Saltveit (2002). Antioxidant enzyme activity including catalase (CAT) was performed according to Maehly and Chance (1959), ascorbate peroxidase (APX) activity was determined according to Chen and Asada (1989) with minor modification, and guaiacol peroxidase (GPX) activity was determined according to Upadhyaya et al. 1985.
Statistical analysis
The experiment was performed as a factorial based on completely randomized design with three replicates. One-way analysis of variance (ANOVA) was done and means compared using Duncan’s multiple range test at the 99% certainty level (P ≤ 0.01) using SAS 9.1 software.
Results and Discussion
Induction and establishment of hairy root cultures
Seeds were germinated after 5 days (Fig. S1A). The cotyledon explants, from 1-wk-old seedlings of H. reticulatus (Fig. S1B) were isolated (Fig. S1C) and infected with A. rhizogenes strain A7 (Fig. S1D). After 2 weeks, hairy roots were induced and appeared (Figs. S2A, S2B). Normal and rapid grown hairy roots in solidified MS media (Figs. S2C, S2D) were selected to establish hairy root lines in liquid MS media. Line 8 (L8) with normal morphological structures and stable growth was selected for the next experiments (Fig. S2E). Hairy roots were harvested a week after treatment for alkaloid extraction (Fig. S2F).
PCR analysis for molecular confirmation of transformation
To probe the existence of the rol B gene conveyed from A. rhizogenes Ri plasmid, PCR analysis was conducted. Figure 2 shows PCR assay for identification of the rol B gene in two acquired hairy root lines of H. reticulatus. The PCR analysis of hairy roots produced an amplicon as well as the positive control, while no amplicon observed in the DNA extracted from H. reticulatus roots and negative control.
FeNP effects on hairy root growth and tropane alkaloid production
ANOVA showed that the growth of H. reticulatus hairy roots had not been significantly affected by different exposure times and concentrations of FeNPs (Supplementary material, Table 1, P ≤ 0.01). The highest hairy root fresh and dry weights were found in the medium supplemented with 900 mg L−1 FeNPs (10.56 and 0.61 g, respectively). However, there were no significant differences among fresh and dry weights of treated hairy roots and control (9.25 and 0.52 g, respectively). Extracted materials were used for GC-MS analysis. (Fig. 3 and Figs. S3A, S3B).
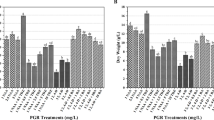
GC-MS analysis revealed that elicitation with FeNPs at different concentrations and exposure times significantly affected content of hyoscyamine (Fig. 4 a) and scopolamine (Fig. 4 b) in hairy root cultures. The maximum hyoscyamine production was obtained in cultures subjected to 900 mg L−1 FeNPs for 24 h (43.82 vs. 8.69% in the control cultures, about fivefold increase). Elicitation with the highest FeNP concentration (3600 mg L−1) for 24 h resulted in minimum hyoscyamine production. The maximum scopolamine accumulation (20.3%) was observed in cultures elicited with 450 mg L−1 FeNPs for 48 h. The quantity of scopolamine in elicitated hairy roots with 450 and 3600 mg L−1 FeNPs for 72 h was decreased to 0.32 and 0.40%, respectively, compared to the scopolamine amount in the non-elicitated sample (4.27%).
Effects of different concentrations of iron oxide nanoparticles at different exposure times on hyoscyamine (a) and scopolamine (b) content in hairy root culture of Hyoscyamus reticulatus. Mean values marked with different letters are significantly different according Duncan’s multiple range test (P ≤ 0.01). Error bars for standard errors (SE), n = 3.
The results showed that increasing exposure time significantly reduced hyoscyamine and scopolamine production. Increasing the treatment period decreased the secondary metabolite production, due to the toxic effects of nanoparticles on mitotic index (genotoxic) and DNA (Castiglione et al. 2011). Increasing the concentration of iron oxide nanoparticles resulted in a decline in tropane alkaloid production. The toxic effects of high concentrations of nanoparticles have been reported by several researchers (Yang and Watts 2005; Lin and Xing 2008; Sharafi et al. 2013 b).
The results demonstrated that hyoscyamine and scopolamine contents elicited in hairy roots with appropriate concentrations and exposure times were higher than the control. The results showed that iron oxide nanoparticles stimulated hyoscyamine and scopolamine production in H. reticulatus hairy root culture.
The last part of the tropane biosynthetic pathway is due to hyoscyamine-6-β-hydroxylase, which catalyzes the hydroxylation of hyoscyamine to scopolamine in two steps (Hashimoto and Yamada 1987). It seems that elicitation of H. reticulatus hairy root culture with iron oxide nanoparticles could make available sufficient Fe2+ required for this enzymatic reaction and increase the production of tropane alkaloids. Iron nanooxide is a novel elicitor of which there is no report available regarding utilization in hairy root culture of H. reticulatus. Nanoparticles on account of their physicochemical properties, e.g., enlarged surface area to volume, high surface reactivity, and ability to engineer electron exchange, can affect the redox status and modify the growth efficiency of plants (Mukherjee and Mahapatra 2009). For increasing tropane alkaloids, various techniques such as genetic engineering of key enzymes in biosynthetic pathway were analyzed. For example, engineered belladonna hairy roots with transgenic hyoscyamine-6β-hydroxylase gene recorded a fivefold-increased scopolamine production compared to native roots (Hashimoto et al. 1993). Over-expression of pmt and h6h gene in Atropa belladonna L. caused a huge increase (11 and 24 times) in hyoscyamine content in elicitated hairy roots compared to control and native roots, respectively (Yang et al. 2011).
The scopolamine levels in root cultures of H. niger after addition of 0.5 and 1 g L−1 yeast extract were increased (Hong et al. 2012). In D. metel hairy root culture, atropine content increased 2.4-fold after 48 h elicitation by nanosilver (Shakeran et al. 2015).
Activating specific genes and synthesis of alkaloids depends on various signaling molecules which interact with their related receptors in the plant plasma membrane. Biological or non-biological agents, used as elicitors, are responsible for triggering defense-related compounds through activation of specific transcription factors involved in secondary metabolite production. Jasmonate (JA) is one of the most important growth regulators which stimulate diverse plant defense responses, including the biosynthesis of secondary metabolites. It seems that nanoparticles may act in signal transduction paths that promote jasmonate production genes in cells under treatment (Sharafi et al. 2013 a).
Biochemical and GC-MS results revealed that elicitation by iron oxide nanoparticles had significant effects on the activity of key enzymes of tropane alkaloid biosynthesis such as putrescine N-methyltransferase (PMT) and hyoscyamine 6-β-hydroxylase (H6H). Also, elicitation directly or indirectly increased the pmt and the h6h gene expression leading to stimulation of tropane alkaloid production in hairy root cultures.
This study is the first report of FeNP application in hairy root culture of medicinal plants. Many of available reports about the in vitro application of nanoparticles relate to silver and other nanoparticles. The results of Sharafi et al. (2013 b) indicated an effective role of FeNPs in hypericine and hyperforine enhancement in cell suspension culture of H. perforatum L. Publications show that silver nanoparticles have an effective role in promotion of artemisinin producing in A. annua (Zhang et al. 2013), atropine in D. metel (Shakeran et al. 2015), and taxol in hazel cell suspension culture (Jamshidi et al. 2014). Cobalt and zinc nanoparticles increased the expression of genes related to the artemisinin biosynthetic pathway and have been proposed as elicitors to increase artemisinin content. Treatment of G. glabra L. seedlings with CuO and ZnO nanoparticles increased glycyrrhizin contents (Oloumi et al. 2015). Also, nanosized titanium dioxide had positive effects on tropane alkaloid production in H. niger L. plants. The results of this current study confirmed the enhanced production of hyoscyamine and scopolamine in H. reticulatus Lhairy root culture, elicited by FeNPs, and are in accordance with the results of research detailed above.
Effect of FeNPs on antioxidant enzyme activity of H. reticulatus hairy root cultures
Antioxidant enzyme activity was significantly increased in induced hairy roots compared to non-transgenic roots (Supplementary material, Table 2). The results revealed that elicitation of hairy root cultures with FeNPs at different concentrations and exposure times significantly (P ≤ 0.01) affected CAT and GPX activity, while there was no notable difference in the function of APX. Significant variations in antioxidant enzymes activity between the elicited hairy roots were detected. The highest CAT and GPX activity was detected in hairy root cultures exposed to 900 mg L−1 FeNPs for 24 and 48 h, respectively, and the lowest activity of both enzymes obtained after elicitation with 450 mg L−1 FeNPs for 72 h (Fig. 5 a, b).
Effects of different concentrations of iron oxide nanoparticles at different exposure times on catalase (a) and guaiacol peroxidase (b) activity in hairy root culture of Hyoscyamus reticulatus. Mean values marked with different letters are significantly different according Duncan’s multiple range test (P ≤ 0.01). Error bars for standard errors (SE), n = 3.
Ascorbate is a substrate of APX in the final steps of the tropane alkaloid biosynthetic pathway (Fig. 1). As a result, APX activity was not significantly affected by elicitation. Elicitation by iron oxide nanoparticles lead to induction of oxidative stress. Most secondary metabolites from medicinal plants are defensive metabolites and can be stimulated by various elicitors. Hence, production of ROS by FeNPs as elicitor can lead to increased production of tropane alkaloids.
Conclusion
The results of this study proved that use of iron oxide nanoparticles as abiotic elicitor was an effective method for enhancement of tropane alkaloids. According to the results, exposure of hairy root cultures of H. reticulatus to 900 mg L−1 FeNPs for 24 h and 450 mg L−1 FeNPs for 48 h was the best treatments for enhancement of hyoscyamine and scopolamine, respectively. This study is the first report of the application of FeNPs in hairy root culture. Results of these and other studies on nanosized particles demonstrated enhancement of secondary metabolite production. It seems that use of nanoparticles as abiotic elicitors could be an effective strategy to increase productivity of pharmaceutical compounds in medicinal plants.
References
Castiglione MR, Giorgetti L, Geri C, Cremonini R (2011) The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J Nanopart Res 13:2443–2449
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Gharari H, Farjaminezhad M, Marefat A, Fakhari AR (2016) All-in one solid-phase microextraction: development of a selective solid-phase microextraction fiber assembly for the simultaneous and efficient extraction of analytes with different polarities. J Sep Sci 39:1709–1716
Ghorbanpour M, Hatami M, Hatami M (2015) Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nano-sized titanium dioxide and bulk application. Acta Agric Slov 105:23–32
Hashimoto T, Yamada Y (1987) Purification and characterization of hyoscyamine 6β-hydroxylase from root culture of Hyoscyamus niger L. Eur J Biochem 194:277–285
Hashimoto T, Yun D, Yamada Y (1993) Production of tropane alkaloids in genetically engineered root cultures. Phytochem 32:713–718
Hatami M, Ghorbanpour M (2014) Defense enzymes activity and biochemical variations of Pelargonium zonale in response to nanosilver particles and dark storage. Turk J Biol 38:130–139
Hong MLK, Bhatt A, Ping NS, Keng CL (2012) Detection of elicitation effect on Hyoscyamus niger L. root cultures for the root growth and production of tropane alkaloids. Rom Biotech Lett 17:7340–7351
Jamshidi M, Ghanati F, Rezaei A, Bemani E (2014) Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology doi: 10.1007/s10616–014-9808-y
Jaremicz Z, Luczkiewicz M, Kokotkiewicz A, Krolicka A, Sowinski P (2013) Production of tropane alkaloids in Hyoscyamus niger (black henbane) hairy roots grown in bubble-column and spray bioreactors. Biotechnol Lett doi: 10.1007/s10529–013–1426-9
Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teeri TH, Oksman-Caldentey KM (1999) Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 208:545–551
Kai G, Yang S, Zhang Y, Luo X, Fu X, Zhang A, Xiao J (2012) Effects of different elicitors on yield of tropane alkaloids in hairy roots of Anisodus acutangulus. Mol Biol Rep 39:1721–1729
Kamada H, Okamura N, Satake M, Harada H, Shimomura K (1986) Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep 5:239–242
Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576
Lin D, Xing B (2008) Root uptake and phytotoxicity of Zn nanoparticles. IJEST 42:5580–5582
Madani H, Hosseini B, Dehghan E, Rezaei-chiyaneh E (2015) Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L. Acta Physiol Plant 37:55
Maehly AC, Chance B (1959) The assay of catalase and peroxidase. In: Glick D (ed) Methods of biochemical analysis, vol 1. Interscience Publishers, New York, pp 357–425
Mirzaee H, Sharafi A, Hashemi Sohi H (2016) In vitro regeneration and transient expression of sesquiterpene cyclase (SQC) in Artemisia annua L. S Afr J Bot 104:225–231
Mukherjee M, Mahapatra A (2009) Effect of coinage metal nanoparticles and zwitterionic surfactant on reduction of [Co(NH3)5Cl](NO3)2 by iron. Colloids and Surfaces 350:1–7
Oloumi H, Soltaninejad R, Baghizadeh A (2015) The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L.seedlings. Indian J Plant Physiol doi:10.1007/s40502–015-0143-x
Pitta-Alvarez SI, Spollansky TC, Giulietti AM (2000) The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym Microb Technol 26:252–258
Qin B, Ma L, Wang Y, Chen M, Lan X, Wu N, Liao Z (2014) Effects of acetylsalicylic acid and UV-B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus. Plant Cell Tiss and Org Cult 117:483–490
Shakeran Z, Keyhanfar M, Asghari G, Ghanadian M (2015) Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk J Biol 39:111–118
Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Dehsara B, Khalifani BH (2013a) Enhanced morphinan alkaloid production in hairy root cultures of Papaver bracteatum by over-expression of salutaridinol 7-o-acetyltransferase gene via Agrobacterium rhizogenes mediated transformation. World J Microbiol Biotechnol 29:2125–2131
Sharafi A, Sohi HH, Azadi P, Sharafi AA (2014a) Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants 20:257–262
Sharafi A, Sohi HH, Mirzaee H, Azadi P (2014b) In vitro regeneration and Agrobacterium mediated genetic transformation of Artemisia aucheri Boiss. Physiol Mol Biol Plants 20:487–494
Sharafi E, Khayam Nekoei SM, Fotokian MH, Davoodi D, Hadavand Mirzaei H, Hasanloo T (2013b) Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St John’s wort (Hypericum perforatum L.). JMPB 2:177–184
Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith BN (1985) Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol 121:453–461
Valimehr S, Sanjarian F, Sohi HH, Sabouni F, Sharafi A (2014) A reliable and efficient protocol for inducing genetically transformed roots in medicinal plant Nepeta pogonosperma. Physiol Mol Biol Plants 20:351–356
Yang C, ChenM, Zeng L, Zhang L, Liu X, Lan X, Tang K, Liao Z (2011)Improvement of tropane alkaloids production in hairy root cultures of Atropa belladonna by overexpressing pmt and h6h genes. Plant omics 4:29–33
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in hytotoxicity of alumina nanoparticles. Toxicol Lett 158:122–123
Zhang B, Zheng LP, Li WY, Wang JW (2013) Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr Nanosci 9:363–370
Acknowledgments
We acknowledge the staff of Horticulture Department Laboratory, Faculty of Agriculture, Urmia University, for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
The original version of this article was revised: The correct names of the first and second authors are Fereshte Moharrami and Bahman Hosseini, respectively.
An erratum to this article is available at http://dx.doi.org/10.1007/s11627-017-9821-x.
Electronic supplementary material
ESM 1
(DOC 3381 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moharrami, F., Hosseini, B., Sharafi, A. et al. Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell.Dev.Biol.-Plant 53, 104–111 (2017). https://doi.org/10.1007/s11627-017-9802-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9802-0